[English] 日本語
 Yorodumi
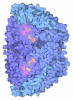
Yorodumi- PDB-5n28: METHYL-COENZYME M REDUCTASE III FROM METHANOTORRIS FORMICICUS MON... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5n28 | ||||||
|---|---|---|---|---|---|---|---|
| Title | METHYL-COENZYME M REDUCTASE III FROM METHANOTORRIS FORMICICUS MONOCLINIC FORM | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE / POST-TRANSLATIONAL MODIFICATION / BINDING SITES / CATALYSIS / COENZYMES / DISULFIDES / HYDROGEN / HYDROGEN BONDING / LIGANDS / MESNA / METALLOPORPHYRINS / METHANE / METHANOCOCCALES / NICKEL / OXIDATION-REDUCTION / OXIDOREDUCTASES / PHOSPHOTHREONINE / PROTEIN CONFORMATION / PROTEIN FOLDING / PROTEIN STRUCTURE / THERMOPHILE / AUTOTROPH / HYDROXY-TRYPTOPHANE | ||||||
| Function / homology |  Function and homology information Function and homology informationcoenzyme-B sulfoethylthiotransferase / coenzyme-B sulfoethylthiotransferase activity / methanogenesis / metal ion binding Similarity search - Function | ||||||
| Biological species |  Methanotorris formicicus Mc-S-70 (archaea) Methanotorris formicicus Mc-S-70 (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Wagner, T. / Wegner, C.E. / Ermler, U. / Shima, S. | ||||||
 Citation Citation |  Journal: J.Bacteriol. / Year: 2017 Journal: J.Bacteriol. / Year: 2017Title: Phylogenetic and Structural Comparisons of the Three Types of Methyl Coenzyme M Reductase from Methanococcales and Methanobacteriales. Authors: Wagner, T. / Wegner, C.E. / Kahnt, J. / Ermler, U. / Shima, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5n28.cif.gz 5n28.cif.gz | 981.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5n28.ent.gz pdb5n28.ent.gz | 817.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5n28.json.gz 5n28.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/n2/5n28 https://data.pdbj.org/pub/pdb/validation_reports/n2/5n28 ftp://data.pdbj.org/pub/pdb/validation_reports/n2/5n28 ftp://data.pdbj.org/pub/pdb/validation_reports/n2/5n28 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5n1qC  5n2aC  5a8wS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AD
| #1: Protein | Mass: 61180.930 Da / Num. of mol.: 2 / Source method: isolated from a natural source Details: IN CHAIN A AND D RESIDUE 260 IS A N1-METHYLHISTIDINE. RESIDUE 274 IS A C5-(S)-METHYLARGININE. RESIDUE 402 IS A C2-(S)-METHYLGLUTAMINE. RESIDUE 429 IS A POSSIBLE 6-HYDROXY-TRYPTOPHANE. ...Details: IN CHAIN A AND D RESIDUE 260 IS A N1-METHYLHISTIDINE. RESIDUE 274 IS A C5-(S)-METHYLARGININE. RESIDUE 402 IS A C2-(S)-METHYLGLUTAMINE. RESIDUE 429 IS A POSSIBLE 6-HYDROXY-TRYPTOPHANE. RESIDUE 447 IS A THIOGLYCINE. Source: (natural)  Methanotorris formicicus Mc-S-70 (archaea) Methanotorris formicicus Mc-S-70 (archaea)Cell line: / / Organ: / / Plasmid details: DSMZ / Variant: Wild-type / Tissue: / References: UniProt: H1KXL5, coenzyme-B sulfoethylthiotransferase |
|---|
-Methyl-coenzyme M reductase, ... , 2 types, 4 molecules BECF
| #2: Protein | Mass: 47541.703 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Methanotorris formicicus Mc-S-70 (archaea) Methanotorris formicicus Mc-S-70 (archaea)Cell line: / / Organ: / / Plasmid details: DSMZ / Variant: Wild-type / Tissue: / References: UniProt: H1KXL9, coenzyme-B sulfoethylthiotransferase #3: Protein | Mass: 30274.383 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Methanotorris formicicus Mc-S-70 (archaea) Methanotorris formicicus Mc-S-70 (archaea)Cell line: / / Organ: / / Plasmid details: DSMZ / Variant: Wild-type / Tissue: / References: UniProt: H1KXL6, coenzyme-B sulfoethylthiotransferase |
|---|
-Non-polymers , 5 types, 35 molecules 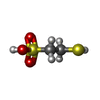
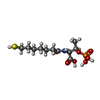
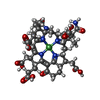






| #4: Chemical | | #5: Chemical | #6: Chemical | #7: Chemical | ChemComp-K / | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.27 Å3/Da / Density % sol: 45.8 % / Description: Yellow rod-shaped crystals |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 2 ul of MCR III from M. formicicus (35 mg/ml) and 1 ul of reservoir solution containing 20% PEG 8000 (w/v), 100 mM MES pH 7.0. The cryoprotection solution contains 20% PEG 8000 (w/v), 100 mM ...Details: 2 ul of MCR III from M. formicicus (35 mg/ml) and 1 ul of reservoir solution containing 20% PEG 8000 (w/v), 100 mM MES pH 7.0. The cryoprotection solution contains 20% PEG 8000 (w/v), 100 mM MES pH 7.0, 25% ethylene glycol (v/v). PH range: / / Temp details: / |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 0.99998 Å / Beamline: X10SA / Wavelength: 0.99998 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: May 22, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.99998 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→49.36 Å / Num. obs: 60676 / % possible obs: 99.9 % / Redundancy: 5 % / Biso Wilson estimate: 79.71 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.103 / Rpim(I) all: 0.05 / Net I/σ(I): 10.8 |
| Reflection shell | Resolution: 2.8→2.95 Å / Redundancy: 5 % / Rmerge(I) obs: 0.755 / Mean I/σ(I) obs: 2.1 / Num. unique all: 8783 / Num. unique obs: 8783 / CC1/2: 0.317 / Rpim(I) all: 0.371 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: MCRII from Methanothermobacter wolfeii PDB:5A8W Resolution: 2.8→48.43 Å / Cor.coef. Fo:Fc: 0.9471 / Cor.coef. Fo:Fc free: 0.9445 / Cross valid method: THROUGHOUT / σ(F): 0 / SU Rfree Blow DPI: 0.312
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 94.18 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.477 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.8→48.43 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.8→2.87 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj