[English] 日本語
 Yorodumi
Yorodumi- PDB-5fpo: Structure of Bacterial DNA Ligase with small-molecule ligand 1H- ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5fpo | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Bacterial DNA Ligase with small-molecule ligand 1H- indazol-7-amine (AT4213) in an alternate binding site. | ||||||
 Components Components | DNA LIGASE | ||||||
 Keywords Keywords | LIGASE / ANTIBIOTIC DESIGN / PROTEIN-LIGAND COMPLEX / FRAGMENT SCREENING / ALTERNATE BINDING SITE / AT4213. | ||||||
| Function / homology |  Function and homology information Function and homology informationDNA ligase (NAD+) / DNA ligase (NAD+) activity / DNA replication / DNA repair / DNA binding / metal ion binding / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.83 Å MOLECULAR REPLACEMENT / Resolution: 1.83 Å | ||||||
 Authors Authors | Jhoti, H. / Ludlow, R.F. / Pathuri, P. / Saini, H.K. / Tickle, I.J. / Tisi, D. / Verdonk, M. / Williams, P.A. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2015 Journal: Proc.Natl.Acad.Sci.USA / Year: 2015Title: Detection of Secondary Binding Sites in Proteins Using Fragment Screening. Authors: Ludlow, R.F. / Verdonk, M.L. / Saini, H.K. / Tickle, I.J. / Jhoti, H. #1:  Journal: Acs Med.Chem.Lett. / Year: 2013 Journal: Acs Med.Chem.Lett. / Year: 2013Title: Fragment-Based Discovery of 6-Azaindazoles as Inhibitors of Bacterial DNA Ligase. Authors: Howard, S. / Amin, N. / Benowitz, A.B. / Chiarparin, E. / Cui, H. / Deng, X. / Heightman, T.D. / Holmes, D.J. / Hopkins, A. / Huang, J. / Jin, Q. / Kreatsoulas, C. / Martin, A.C.L. / Massey, ...Authors: Howard, S. / Amin, N. / Benowitz, A.B. / Chiarparin, E. / Cui, H. / Deng, X. / Heightman, T.D. / Holmes, D.J. / Hopkins, A. / Huang, J. / Jin, Q. / Kreatsoulas, C. / Martin, A.C.L. / Massey, F. / Mccloskey, L. / Mortenson, P.N. / Pathuri, P. / Tisi, D. / Williams, P.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5fpo.cif.gz 5fpo.cif.gz | 137.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5fpo.ent.gz pdb5fpo.ent.gz | 108.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5fpo.json.gz 5fpo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fp/5fpo https://data.pdbj.org/pub/pdb/validation_reports/fp/5fpo ftp://data.pdbj.org/pub/pdb/validation_reports/fp/5fpo ftp://data.pdbj.org/pub/pdb/validation_reports/fp/5fpo | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5fp5C  5fp6C  5fpdC  5fpeC  5fpmC  5fpnC  5fprC  5fpsC  5fptC  5fpyC  4cc5S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 36724.789 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q9AIU7, DNA ligase (NAD+), DNA ligase (ATP) | ||||||
|---|---|---|---|---|---|---|---|
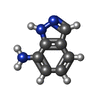
| #2: Chemical | | #3: Water | ChemComp-HOH / | Nonpolymer details | 1H-INDAZOL-7-AMINE (10L): ASTEX COMPOUND REGISTRY AT4213. | Sequence details | DELETION 313-355 C-TERM HIS TAG. | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.43 Å3/Da / Density % sol: 48.96 % / Description: NONE |
|---|---|
| Crystal grow | Details: 1.6M (NH4)2SO4. PROTEIN CONC. = 27.2 MG/ML. |
-Data collection
| Diffraction | Mean temperature: 93 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.9724 / Beamline: ID23-1 / Wavelength: 0.9724 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Aug 25, 2010 / Details: MIRRORS |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9724 Å / Relative weight: 1 |
| Reflection | Resolution: 1.83→48.8 Å / Num. obs: 29257 / % possible obs: 96.7 % / Observed criterion σ(I): -3.7 / Redundancy: 2.3 % / Biso Wilson estimate: 21.58 Å2 / Rmerge(I) obs: 0.09 / Net I/σ(I): 6.4 |
| Reflection shell | Resolution: 1.83→1.92 Å / Rmerge(I) obs: 0.33 / Mean I/σ(I) obs: 2.8 / % possible all: 89.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4CC5 Resolution: 1.83→48.78 Å / Cor.coef. Fo:Fc: 0.8799 / Cor.coef. Fo:Fc free: 0.8891 / SU R Cruickshank DPI: 0.152 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.158 / SU Rfree Blow DPI: 0.143 / SU Rfree Cruickshank DPI: 0.141 / Details: DISORDERED REGIONS WERE DELETED.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.96 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.278 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.83→48.78 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.83→1.9 Å / Total num. of bins used: 14
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj