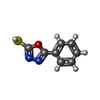[English] 日本語
 Yorodumi
Yorodumi- PDB-5fpm: Structure of heat shock-related 70kDA protein 2 with small-molecu... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5fpm | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of heat shock-related 70kDA protein 2 with small-molecule ligand 5-phenyl-1,3,4-oxadiazole-2-thiol (AT809) in an alternate binding site. | ||||||
 Components Components | HEAT SHOCK-RELATED 70KDA PROTEIN 2 | ||||||
 Keywords Keywords | CHAPERONE / HEAT SHOCK-RELATED PROTEIN / HEAT SHOCK / HSP70 / HSPA2 / PROTEIN-LIGAND COMPLEX / FRAGMENT SCREENING / ALTERNATE BINDING SITE / AT809. | ||||||
| Function / homology |  Function and homology information Function and homology informationCatSper complex / glycolipid binding / synaptonemal complex disassembly / negative regulation of inclusion body assembly / male meiotic nuclear division / synaptonemal complex / meiotic spindle / : / male meiosis I / spermatid development ...CatSper complex / glycolipid binding / synaptonemal complex disassembly / negative regulation of inclusion body assembly / male meiotic nuclear division / synaptonemal complex / meiotic spindle / : / male meiosis I / spermatid development / Regulation of HSF1-mediated heat shock response / positive regulation of G2/M transition of mitotic cell cycle / response to unfolded protein / Attenuation phase / heat shock protein binding / protein folding chaperone / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / response to cold / Meiotic synapsis / male germ cell nucleus / ATP-dependent protein folding chaperone / PKR-mediated signaling / tau protein binding / disordered domain specific binding / unfolded protein binding / protein-folding chaperone binding / response to heat / protein refolding / blood microparticle / spermatogenesis / enzyme binding / cell surface / ATP hydrolysis activity / extracellular exosome / ATP binding / membrane / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.96 Å MOLECULAR REPLACEMENT / Resolution: 1.96 Å | ||||||
 Authors Authors | Jhoti, H. / Ludlow, R.F. / Patel, S. / Saini, H.K. / Tickle, I.J. / Verdonk, M. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2015 Journal: Proc.Natl.Acad.Sci.USA / Year: 2015Title: Detection of Secondary Binding Sites in Proteins Using Fragment Screening. Authors: Ludlow, R.F. / Verdonk, M.L. / Saini, H.K. / Tickle, I.J. / Jhoti, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5fpm.cif.gz 5fpm.cif.gz | 318.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5fpm.ent.gz pdb5fpm.ent.gz | 259.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5fpm.json.gz 5fpm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fp/5fpm https://data.pdbj.org/pub/pdb/validation_reports/fp/5fpm ftp://data.pdbj.org/pub/pdb/validation_reports/fp/5fpm ftp://data.pdbj.org/pub/pdb/validation_reports/fp/5fpm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5fp5C  5fp6C  5fpdSC  5fpeC  5fpnC  5fpoC  5fprC  5fpsC  5fptC  5fpyC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.99999, 0.00174, 0.00515), Vector: |
- Components
Components
| #1: Protein | Mass: 42383.000 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PET28B / Production host: HOMO SAPIENS (human) / Plasmid: PET28B / Production host:  #2: Chemical | #3: Water | ChemComp-HOH / | Nonpolymer details | 5-PHENYL-1,3,4-OXADIAZOLE | Sequence details | 3 EXTRA RESIDUES AT N TERM ARE REMAINS OF HIS TAG AFTER THROMBIN CLEAVAGE. RESIDUES 2-3 DELETED. ...3 EXTRA RESIDUES AT N TERM ARE REMAINS OF HIS TAG AFTER THROMBIN CLEAVAGE. RESIDUES 2-3 DELETED. RESIDUES 386-639 DELETED. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 46.14 % / Description: NONE |
|---|---|
| Crystal grow | pH: 8 Details: 1.0M NACL, 0.1M TRIS/HCL PH=8, 20.0% W/V PEG 8000. PROTEIN CONC. = 11MG/ML. |
-Data collection
| Diffraction | Mean temperature: 93 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU FR-E / Wavelength: 1.5418 |
| Detector | Type: RIGAKU SATURN CCD / Detector: CCD / Date: Jun 24, 2008 / Details: MIRRORS |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.96→38.4 Å / Num. obs: 53541 / % possible obs: 97.8 % / Observed criterion σ(I): -3.7 / Redundancy: 2.2 % / Biso Wilson estimate: 24.64 Å2 / Rmerge(I) obs: 0.06 / Net I/σ(I): 9.9 |
| Reflection shell | Resolution: 1.96→2.03 Å / Rmerge(I) obs: 0.22 / Mean I/σ(I) obs: 2.9 / % possible all: 89.3 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 5FPD Resolution: 1.96→38.37 Å / Cor.coef. Fo:Fc: 0.9491 / Cor.coef. Fo:Fc free: 0.9282 / SU R Cruickshank DPI: 0.177 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.206 / SU Rfree Blow DPI: 0.166 / SU Rfree Cruickshank DPI: 0.156
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 26.63 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.191 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.96→38.37 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.96→2.01 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller












 PDBj
PDBj