[English] 日本語
 Yorodumi
Yorodumi- PDB-4zyb: High resolution structure of M23 peptidase LytM with substrate an... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4zyb | ||||||
|---|---|---|---|---|---|---|---|
| Title | High resolution structure of M23 peptidase LytM with substrate analogue | ||||||
 Components Components | Glycyl-glycine endopeptidase LytM | ||||||
 Keywords Keywords | HYDROLASE / LYTM / LYSOSTAPHIN / PEPTIDOGLYCAN AMIDASE / PEPTIDASE / TETRAGLYCINE PHOSPHINATE / TRANSITION STATE ANALOGUE / COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationlysostaphin / cobalt ion binding / peptide catabolic process / nickel cation binding / cell wall organization / metalloendopeptidase activity / manganese ion binding / proteolysis / extracellular region / zinc ion binding / metal ion binding Similarity search - Function | ||||||
| Biological species |  Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria) Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Grabowska, M. / Jagielska, E. / Czapinska, H. / Bochtler, M. / Sabala, I. | ||||||
| Funding support |  Poland, 1items Poland, 1items
| ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2015 Journal: Sci Rep / Year: 2015Title: High resolution structure of an M23 peptidase with a substrate analogue. Authors: Grabowska, M. / Jagielska, E. / Czapinska, H. / Bochtler, M. / Sabala, I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4zyb.cif.gz 4zyb.cif.gz | 264.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4zyb.ent.gz pdb4zyb.ent.gz | 212.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4zyb.json.gz 4zyb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4zyb_validation.pdf.gz 4zyb_validation.pdf.gz | 1.7 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4zyb_full_validation.pdf.gz 4zyb_full_validation.pdf.gz | 1.7 MB | Display | |
| Data in XML |  4zyb_validation.xml.gz 4zyb_validation.xml.gz | 33.3 KB | Display | |
| Data in CIF |  4zyb_validation.cif.gz 4zyb_validation.cif.gz | 50 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zy/4zyb https://data.pdbj.org/pub/pdb/validation_reports/zy/4zyb ftp://data.pdbj.org/pub/pdb/validation_reports/zy/4zyb ftp://data.pdbj.org/pub/pdb/validation_reports/zy/4zyb | HTTPS FTP |
-Related structure data
| Related structure data |  2b0pS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 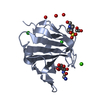
| ||||||||
| 2 | 
| ||||||||
| 3 | 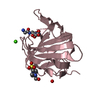
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 14564.811 Da / Num. of mol.: 4 / Fragment: unp residues 185-316 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria) Staphylococcus aureus subsp. aureus NCTC 8325 (bacteria)Gene: lytM, SAS0252 / Plasmid: PLASMID / Details (production host): PET15B / Production host:  References: UniProt: Q6GCJ6, UniProt: O33599*PLUS, lysostaphin |
|---|
-Non-polymers , 9 types, 889 molecules 



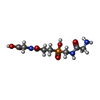










| #2: Chemical | ChemComp-ZN / #3: Chemical | ChemComp-CA / #4: Chemical | ChemComp-CL / #5: Chemical | ChemComp-UNL / Mass: 103.120 Da / Num. of mol.: 4 / Source method: obtained synthetically #6: Chemical | #7: Chemical | ChemComp-4SQ / #8: Chemical | #9: Chemical | ChemComp-PEG / | #10: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.19 Å3/Da / Density % sol: 43.76 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 0.05 M HEPES-CsOH pH 7.5, 0.2 M calcium chloride and 25% polyethylene glycol 4000 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.917 Å / Beamline: 14.1 / Wavelength: 0.917 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Nov 24, 2013 |
| Radiation | Monochromator: KMC-1 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.917 Å / Relative weight: 1 |
| Reflection | Resolution: 1.45→40 Å / Num. obs: 87629 / % possible obs: 99.7 % / Redundancy: 6.8 % / CC1/2: 0.998 / Rmerge(I) obs: 0.121 / Rsym value: 0.112 / Net I/σ(I): 12 |
| Reflection shell | Resolution: 1.45→1.54 Å / Redundancy: 6.5 % / Rmerge(I) obs: 0.706 / Mean I/σ(I) obs: 2.5 / CC1/2: 0.813 / Rsym value: 0.649 / % possible all: 98.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2B0P Resolution: 1.5→36.3 Å / SU ML: 0.12 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 17.15 / Stereochemistry target values: ML Details: The N-terminus of the truncated protein (absent in the full length native LytM) is disordered and has been built in only tentatively. The ligand could be reliably modelled in the C-terminal ...Details: The N-terminus of the truncated protein (absent in the full length native LytM) is disordered and has been built in only tentatively. The ligand could be reliably modelled in the C-terminal and phosphinate part, its N-terminus is most likely disordered, since none of its conformations was clearly prevailing in all four molecules in the asymmetric unit. There are numerous close contacts between solvent molecules in the structure which might either result from their static disorder, or poorly ordered PEG molecules, or low occupancy metal ions all of which have been modelled tentatively in a few cases. Alternatively they may result from crystal defects (crystallites of different symmetries forming the macroscopically observed crystal). The identity of a few ions is unsure - for the technical reasons the unsure Cl- ions were kept as Cl ions whereas the unsure Na ions have been submitted as UNL atoms. For a few serine residues we observe difference density close to the side chain oxygen atom.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→36.3 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj








