+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qql | ||||||
|---|---|---|---|---|---|---|---|
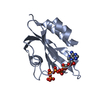
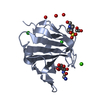
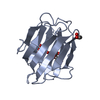
| Title | THE CRYSTAL STRUCTURE OF FIBROBLAST GROWTH FACTOR 7/1 CHIMERA | ||||||
 Components Components | Fibroblast growth factor 7, Fibroblast growth factor 1 chimera | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / BETA-TREFOIL / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationsecretion by lung epithelial cell involved in lung growth / FGFR2b ligand binding and activation / SHC-mediated cascade:FGFR2 / FRS-mediated FGFR2 signaling / type 2 fibroblast growth factor receptor binding / PI-3K cascade:FGFR2 / PI3K Cascade / PIP3 activates AKT signaling / Phospholipase C-mediated cascade; FGFR2 / positive regulation of keratinocyte proliferation ...secretion by lung epithelial cell involved in lung growth / FGFR2b ligand binding and activation / SHC-mediated cascade:FGFR2 / FRS-mediated FGFR2 signaling / type 2 fibroblast growth factor receptor binding / PI-3K cascade:FGFR2 / PI3K Cascade / PIP3 activates AKT signaling / Phospholipase C-mediated cascade; FGFR2 / positive regulation of keratinocyte proliferation / Negative regulation of FGFR2 signaling / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / mesonephric epithelium development / branch elongation involved in ureteric bud branching / regulation of endothelial tube morphogenesis / positive regulation of epithelial cell proliferation involved in lung morphogenesis / FGFR3b ligand binding and activation / regulation of branching involved in salivary gland morphogenesis by mesenchymal-epithelial signaling / regulation of endothelial cell chemotaxis to fibroblast growth factor / Signaling by activated point mutants of FGFR3 / FGFR3c ligand binding and activation / Phospholipase C-mediated cascade; FGFR3 / phosphatidylcholine biosynthetic process / mesenchymal cell proliferation / positive regulation of myoblast proliferation / positive regulation of keratinocyte migration / FGFR2b ligand binding and activation / fibroblast growth factor receptor binding / FGFR2c ligand binding and activation / Activated point mutants of FGFR2 / FGFR4 ligand binding and activation / Phospholipase C-mediated cascade; FGFR2 / FGFR1b ligand binding and activation / Phospholipase C-mediated cascade; FGFR4 / Signaling by activated point mutants of FGFR1 / FGFR1c ligand binding and activation / organ induction / Downstream signaling of activated FGFR1 / phospholipid biosynthetic process / Phospholipase C-mediated cascade: FGFR1 / branching involved in salivary gland morphogenesis / regulation of synapse maturation / surfactant homeostasis / S100 protein binding / protein localization to cell surface / RAF/MAP kinase cascade / activation of protein kinase B activity / hair follicle morphogenesis / Signaling by FGFR2 IIIa TM / PI-3K cascade:FGFR3 / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / positive chemotaxis / PI-3K cascade:FGFR1 / positive regulation of sprouting angiogenesis / chemoattractant activity / positive regulation of MAP kinase activity / positive regulation of cell division / positive regulation of intracellular signal transduction / PI3K Cascade / fibroblast growth factor receptor signaling pathway / anatomical structure morphogenesis / SHC-mediated cascade:FGFR3 / SHC-mediated cascade:FGFR2 / SHC-mediated cascade:FGFR4 / ovarian follicle development / SHC-mediated cascade:FGFR1 / FRS-mediated FGFR3 signaling / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / FRS-mediated FGFR1 signaling / Signaling by FGFR3 in disease / positive regulation of endothelial cell proliferation / Signaling by FGFR2 in disease / Signaling by FGFR1 in disease / neurogenesis / positive regulation of endothelial cell migration / lung development / regulation of cell migration / epithelial cell proliferation / positive regulation of epithelial cell proliferation / growth factor activity / Negative regulation of FGFR3 signaling / Negative regulation of FGFR2 signaling / wound healing / Negative regulation of FGFR4 signaling / Negative regulation of FGFR1 signaling / positive regulation of cholesterol biosynthetic process / GABA-ergic synapse / integrin binding / extracellular matrix / positive regulation of angiogenesis / Constitutive Signaling by Aberrant PI3K in Cancer / PIP3 activates AKT signaling / heparin binding / cellular response to heat / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade / actin cytoskeleton organization / angiogenesis Similarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.3 Å SYNCHROTRON / Resolution: 2.3 Å | ||||||
 Authors Authors | Ye, S. / Luo, Y. / Pelletier, H. / McKeehan, W.L. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2001 Journal: Biochemistry / Year: 2001Title: Structural basis for interaction of FGF-1, FGF-2, and FGF-7 with different heparan sulfate motifs. Authors: Ye, S. / Luo, Y. / Lu, W. / Jones, R.B. / Linhardt, R.J. / Capila, I. / Toida, T. / Kan, M. / Pelletier, H. / McKeehan, W.L. #1:  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: The glycine box: a determinant of specificity for fibroblast growth factor. Authors: Luo, Y. / Lu, W. / Mohamedali, K.A. / Jang, J.H. / Jones, R.B. / Gabriel, J.L. / Kan, M. / McKeehan, W.L. #2:  Journal: Science / Year: 1989 Journal: Science / Year: 1989Title: Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Authors: Finch, P.W. / Rubin, J.S. / Miki, T. / Ron, D. / Aaronson, S.A. #3:  Journal: Nature / Year: 1998 Journal: Nature / Year: 1998Title: Structure of a heparin-linked biologically active dimer of fibroblast growth factor. Authors: DiGabriele, A.D. / Lax, I. / Chen, D.I. / Svahn, C.M. / Jaye, M. / Schlessinger, J. / Hendrickson, W.A. #4:  Journal: Science / Year: 1996 Journal: Science / Year: 1996Title: Heparin structure and interactions with basic fibroblast growth factor. Authors: Faham, S. / Hileman, R.E. / Fromm, J.R. / Linhardt, R.J. / Rees, D.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qql.cif.gz 1qql.cif.gz | 42.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qql.ent.gz pdb1qql.ent.gz | 30.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qql.json.gz 1qql.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qq/1qql https://data.pdbj.org/pub/pdb/validation_reports/qq/1qql ftp://data.pdbj.org/pub/pdb/validation_reports/qq/1qql ftp://data.pdbj.org/pub/pdb/validation_reports/qq/1qql | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 16433.945 Da / Num. of mol.: 1 Fragment: DUAL-FUNCTION CHIMERA BETWEEN RAT FGF-7 ENCODED BY EXON 1 AND 2 AND HUMAN FGF-1 ENCODED BY EXON 3,DUAL-FUNCTION CHIMERA BETWEEN RAT FGF-7 ENCODED BY EXON 1 AND 2 AND HUMAN FGF-1 ENCODED BY EXON 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Genus: Rattus, Homo / Species: , / Strain: , / Description: NONE / Gene: Fgf7, FGF1, FGFA / Plasmid: PGEX-2T / Species (production host): Escherichia coli / Production host:  References: UniProt: Q9ERN5, UniProt: P05230, UniProt: Q02195*PLUS |
|---|---|
| #2: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 46.96 % | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 7 Details: SODIUM PHOSPHATE, POTASSIUM PHOSPHATE, SODIUM SULPHATE, pH 7.0, VAPOR DIFFUSION, SITTING DROP, temperature 298K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||||
| Detector |
| ||||||||||||||||||||
| Radiation |
| ||||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||||
| Reflection | Resolution: 2.3→30 Å / Num. all: 7140 / Num. obs: 7140 / % possible obs: 96.8 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 9 % / Rmerge(I) obs: 0.065 / Net I/σ(I): 28.1 | ||||||||||||||||||||
| Reflection shell | Resolution: 2.3→2.38 Å / Redundancy: 7.8 % / Rmerge(I) obs: 0.299 / % possible all: 95 | ||||||||||||||||||||
| Reflection | *PLUS Lowest resolution: 30 Å / Num. measured all: 64157 | ||||||||||||||||||||
| Reflection shell | *PLUS % possible obs: 95 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.3→6 Å / σ(F): 2 / σ(I): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.843 / Classification: refinement X-PLOR / Version: 3.843 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.3 Å / Lowest resolution: 6 Å / σ(F): 2 / Rfactor obs: 0.237 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller











 PDBj
PDBj












