[English] 日本語
 Yorodumi
Yorodumi- PDB-3oni: Crystal Structure of the second bromodomain of human BRD2 in comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3oni | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the second bromodomain of human BRD2 in complex with the inhibitor JQ1 | ||||||
 Components Components | Bromodomain containing 2, isoform CRA_a | ||||||
 Keywords Keywords | CELL CYCLE / Structural Genomics / Structural Genomics Consortium / SGC / Bromodomain / Inhibition / Probe | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone H3K14ac reader activity / acetylation-dependent protein binding / chromatin looping / RUNX3 regulates p14-ARF / histone H4K5ac reader activity / histone H4K12ac reader activity / positive regulation of T-helper 17 cell lineage commitment / protein localization to chromatin / neural tube closure / nucleosome assembly ...histone H3K14ac reader activity / acetylation-dependent protein binding / chromatin looping / RUNX3 regulates p14-ARF / histone H4K5ac reader activity / histone H4K12ac reader activity / positive regulation of T-helper 17 cell lineage commitment / protein localization to chromatin / neural tube closure / nucleosome assembly / histone binding / spermatogenesis / nuclear speck / chromatin remodeling / protein serine/threonine kinase activity / chromatin binding / regulation of transcription by RNA polymerase II / chromatin / nucleoplasm / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.61 Å molecular replacement / Resolution: 1.61 Å | ||||||
 Authors Authors | Filippakopoulos, P. / Picaud, S. / Fedorov, O. / von Delft, F. / Arrowsmith, C.H. / Edwards, A.M. / Weigelt, J. / Bountra, C. / Bradner, J.E. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Nature / Year: 2010 Journal: Nature / Year: 2010Title: Selective inhibition of BET bromodomains. Authors: Filippakopoulos, P. / Qi, J. / Picaud, S. / Shen, Y. / Smith, W.B. / Fedorov, O. / Morse, E.M. / Keates, T. / Hickman, T.T. / Felletar, I. / Philpott, M. / Munro, S. / McKeown, M.R. / Wang, ...Authors: Filippakopoulos, P. / Qi, J. / Picaud, S. / Shen, Y. / Smith, W.B. / Fedorov, O. / Morse, E.M. / Keates, T. / Hickman, T.T. / Felletar, I. / Philpott, M. / Munro, S. / McKeown, M.R. / Wang, Y. / Christie, A.L. / West, N. / Cameron, M.J. / Schwartz, B. / Heightman, T.D. / La Thangue, N. / French, C.A. / Wiest, O. / Kung, A.L. / Knapp, S. / Bradner, J.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3oni.cif.gz 3oni.cif.gz | 66 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3oni.ent.gz pdb3oni.ent.gz | 46.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3oni.json.gz 3oni.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/on/3oni https://data.pdbj.org/pub/pdb/validation_reports/on/3oni ftp://data.pdbj.org/pub/pdb/validation_reports/on/3oni ftp://data.pdbj.org/pub/pdb/validation_reports/on/3oni | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3mxfC 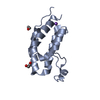 2nxbS  2oo1S  2ossS  2ouoS  2rfjS  3d7cS  3daiS  3dwyS  3hmhS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 13375.410 Da / Num. of mol.: 1 / Fragment: residues 224-335 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: BRD2, DKFZp313H139, hCG_17503 / Plasmid: pNIC28-Bsa4 / Production host: Homo sapiens (human) / Gene: BRD2, DKFZp313H139, hCG_17503 / Plasmid: pNIC28-Bsa4 / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Chemical | ChemComp-JQ1 / ( | ||||
| #3: Chemical | | #4: Chemical | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.25 Å3/Da / Density % sol: 45.43 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 30% Jeffamine600, 0.5M CsCl, 5% glycerol, pH 6.5, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.542 Å ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.542 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Mar 22, 2010 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.542 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Redundancy: 3.4 % / Av σ(I) over netI: 10 / Number: 54515 / Rsym value: 0.055 / D res high: 1.609 Å / D res low: 27.32 Å / Num. obs: 16213 / % possible obs: 99.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diffraction reflection shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.61→29.16 Å / Num. all: 16295 / Num. obs: 16213 / % possible obs: 99.5 % / Redundancy: 3.4 % / Biso Wilson estimate: 14 Å2 / Rmerge(I) obs: 0.055 / Rsym value: 0.055 / Net I/σ(I): 16.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR | Rfactor: 29.42 / Model details: Phaser MODE: MR_AUTO
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: Ensemble of 3HMH, 2NXB, 2OO1, 2OSS, 2OUO, 2RFJ, 3DAI, 3D7C, 3DWY Resolution: 1.61→29.16 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.955 / WRfactor Rfree: 0.1778 / WRfactor Rwork: 0.145 / Occupancy max: 1 / Occupancy min: 0.3 / FOM work R set: 0.9219 / SU B: 1.988 / SU ML: 0.039 / SU R Cruickshank DPI: 0.0793 / SU Rfree: 0.0822 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.082 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES: WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 62.26 Å2 / Biso mean: 13.0347 Å2 / Biso min: 4.44 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.61→29.16 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.61→1.652 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj