[English] 日本語
 Yorodumi
Yorodumi- PDB-2fyn: Crystal Structure Analysis of the double mutant Rhodobacter Sphae... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2fyn | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure Analysis of the double mutant Rhodobacter Sphaeroides bc1 complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | OXIDOREDUCTASE / Transmembrane helices / functional dimer | ||||||
| Function / homology |  Function and homology information Function and homology informationrespiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / electron transfer activity / heme binding / metal ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  Rhodobacter sphaeroides (bacteria) Rhodobacter sphaeroides (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.2 Å MOLECULAR REPLACEMENT / Resolution: 3.2 Å | ||||||
 Authors Authors | Esser, L. / Xia, D. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.Usa / Year: 2006 Journal: Proc.Natl.Acad.Sci.Usa / Year: 2006Title: Surface-modulated motion switch: Capture and release of iron-sulfur protein in the cytochrome bc1 complex. Authors: Esser, L. / Gong, X. / Yang, S. / Yu, L. / Yu, C.A. / Xia, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2fyn.cif.gz 2fyn.cif.gz | 1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2fyn.ent.gz pdb2fyn.ent.gz | 850.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2fyn.json.gz 2fyn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fy/2fyn https://data.pdbj.org/pub/pdb/validation_reports/fy/2fyn ftp://data.pdbj.org/pub/pdb/validation_reports/fy/2fyn ftp://data.pdbj.org/pub/pdb/validation_reports/fy/2fyn | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2fyuC  1qcrS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | the asymmetric unit contains three independent dimers (biological assembly) |
- Components
Components
-Protein , 3 types, 18 molecules ADGJMPBEHKNQCFILOR
| #1: Protein | Mass: 50157.539 Da / Num. of mol.: 6 / Fragment: cytochrome b / Mutation: S287R Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter sphaeroides (bacteria) / Gene: petB, fbcB / Production host: Rhodobacter sphaeroides (bacteria) / Gene: petB, fbcB / Production host:  Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02761 Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02761#2: Protein | Mass: 29373.953 Da / Num. of mol.: 6 / Fragment: cytochrome c1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter sphaeroides (bacteria) / Gene: petC, fbcC / Production host: Rhodobacter sphaeroides (bacteria) / Gene: petC, fbcC / Production host:  Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02760 Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02760#3: Protein | Mass: 19916.322 Da / Num. of mol.: 6 / Fragment: Rieske Iron sulfur protein / Mutation: V135S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter sphaeroides (bacteria) / Gene: petA, fbcF / Production host: Rhodobacter sphaeroides (bacteria) / Gene: petA, fbcF / Production host:  Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02762, quinol-cytochrome-c reductase Rhodobacter sphaeroides (bacteria) / References: UniProt: Q02762, quinol-cytochrome-c reductase |
|---|
-Non-polymers , 4 types, 36 molecules 
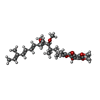





| #4: Chemical | ChemComp-HEM / #5: Chemical | ChemComp-SMA / #6: Chemical | ChemComp-LOP / ( #7: Chemical | ChemComp-FES / |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.38 Å3/Da / Density % sol: 63.6 % |
|---|---|
| Crystal grow | Temperature: 288.2 K / Method: evaporation / pH: 7.5 Details: 10% PEG400, 0.2 M NaCl, 0.2 M Histidine, 0.1M Tris, 10% Glycerol, 5 mM NaN3, 10% Glycerol, 2mM DHPC, 0.5% beta-octyl glucopyranoside, 0.06% sucrose monocarprate, 10mM Sr(NO3)2, pH 7.5, ...Details: 10% PEG400, 0.2 M NaCl, 0.2 M Histidine, 0.1M Tris, 10% Glycerol, 5 mM NaN3, 10% Glycerol, 2mM DHPC, 0.5% beta-octyl glucopyranoside, 0.06% sucrose monocarprate, 10mM Sr(NO3)2, pH 7.5, EVAPORATION, temperature 288.2K |
-Data collection
| Diffraction |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||
| Detector |
| ||||||||||||||||||
| Radiation |
| ||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | ||||||||||||||||||
| Reflection | Resolution: 3→50 Å / Num. all: 160039 / Num. obs: 155528 / % possible obs: 94.3 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -1 / Redundancy: 3.7 % / Rmerge(I) obs: 0.127 / Net I/σ(I): 8.4 | ||||||||||||||||||
| Reflection shell | Resolution: 3→3.11 Å / Redundancy: 2.2 % / Rmerge(I) obs: 0.391 / Mean I/σ(I) obs: 1.37 / Num. unique all: 13841 / % possible all: 94.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1qcr Resolution: 3.2→18 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 111061.03 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 19.4667 Å2 / ksol: 0.285271 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 66 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.2→18 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.2→3.4 Å / Rfactor Rfree error: 0.02 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj
















