+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1g96 | ||||||
|---|---|---|---|---|---|---|---|
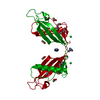
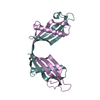
| Title | HUMAN CYSTATIN C; DIMERIC FORM WITH 3D DOMAIN SWAPPING | ||||||
 Components Components | CYSTATIN C | ||||||
 Keywords Keywords | hydrolase inhibitor / human cystatin C dimer / 3D domain swapping / amyloid formation / inhibitor of C1 and C13 cysteine proteases / AMYLOID ANGIOPATHY AND CEREBRAL HEMORRHAGE | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of collagen catabolic process / negative regulation of elastin catabolic process / negative regulation of peptidase activity / negative regulation of blood vessel remodeling / peptidase inhibitor activity / negative regulation of extracellular matrix disassembly / regulation of tissue remodeling / endopeptidase inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of proteolysis ...negative regulation of collagen catabolic process / negative regulation of elastin catabolic process / negative regulation of peptidase activity / negative regulation of blood vessel remodeling / peptidase inhibitor activity / negative regulation of extracellular matrix disassembly / regulation of tissue remodeling / endopeptidase inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of proteolysis / supramolecular fiber organization / Post-translational protein phosphorylation / defense response / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / tertiary granule lumen / amyloid-beta binding / protease binding / vesicle / ficolin-1-rich granule lumen / immune response / endoplasmic reticulum lumen / Amyloid fiber formation / Neutrophil degranulation / endoplasmic reticulum / Golgi apparatus / extracellular space / extracellular exosome / extracellular region / identical protein binding / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Janowski, R. / Kozak, M. / Jankowska, E. / Grzonka, Z. / Grubb, A. / Abrahamson, M. / Jaskolski, M. | ||||||
 Citation Citation |  Journal: Nat.Struct.Biol. / Year: 2001 Journal: Nat.Struct.Biol. / Year: 2001Title: Human cystatin C, an amyloidogenic protein, dimerizes through three-dimensional domain swapping. Authors: Janowski, R. / Kozak, M. / Jankowska, E. / Grzonka, Z. / Grubb, A. / Abrahamson, M. / Jaskolski, M. #1:  Journal: Acta Crystallogr.,Sect.D / Year: 1999 Journal: Acta Crystallogr.,Sect.D / Year: 1999Title: Expression of selenomethionyl derivative and preliminary crystallographic studies of human cystatin C Authors: Kozak, M. / Jankowska, E. / Janowski, R. / Grzonka, Z. / Grubb, A. / Alvarez Fernandez, M. / Abrahamson, M. / Jaskolski, M. #2:  Journal: Embo J. / Year: 1988 Journal: Embo J. / Year: 1988Title: The 2.0 angstrom X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteases Authors: Bode, W. / Engh, R. / Musil, D. / Thiele, U. / Huber, R. / Karshikov, A. / Brzin, J. / Kos, J. / Turk, V. #3:  Journal: J.Mol.Biol. / Year: 1997 Journal: J.Mol.Biol. / Year: 1997Title: NMR structural studies of human cystatin C dimers and monomers Authors: Ekiel, I. / Abrahamson, M. / Fulton, D.B. / Lindahl, P. / Storer, A.C. / Levadoux, W. / Lafrance, M. / Labelle, S. / Pomerleau, Y. / Groleau, D. / LeSauter, L. / Gehring, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1g96.cif.gz 1g96.cif.gz | 36.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1g96.ent.gz pdb1g96.ent.gz | 25.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1g96.json.gz 1g96.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g9/1g96 https://data.pdbj.org/pub/pdb/validation_reports/g9/1g96 ftp://data.pdbj.org/pub/pdb/validation_reports/g9/1g96 ftp://data.pdbj.org/pub/pdb/validation_reports/g9/1g96 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1cewS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||
| 2 | x 8
| ||||||||||
| Unit cell |
| ||||||||||
| Components on special symmetry positions |
| ||||||||||
| Details | Human cystatin C in the present structure forms crystallographic dimers with 3D swapped domains. The dimer is generated by two-fold rotation of the 4(2) axis using the following transformation: 1/2-x, -y, z. |
- Components
Components
| #1: Protein | Mass: 13365.141 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Plasmid: PHD 313 / Production host: Homo sapiens (human) / Plasmid: PHD 313 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-CL / |
| #3: Chemical | ChemComp-GOL / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.3 Å3/Da / Density % sol: 71 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 4.8 Details: Lyophilized protein was dissolved in 100 mM sodium acetate buffer pH 4.8 containing 20 mM CaCl2. Droplets were equilibrated against 1 ml of reservoir with analogous buffer/CaCl2 solution. ...Details: Lyophilized protein was dissolved in 100 mM sodium acetate buffer pH 4.8 containing 20 mM CaCl2. Droplets were equilibrated against 1 ml of reservoir with analogous buffer/CaCl2 solution. After 2 and 4 days, the reservoir solution was supplemented with 100 microliters of MPD, VAPOR DIFFUSION, HANGING DROP, temperature 277K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7B / Wavelength: 0.8423 Å / Beamline: BW7B / Wavelength: 0.8423 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Sep 30, 2000 |
| Radiation | Monochromator: monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8423 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→40 Å / Num. all: 8429 / Num. obs: 8429 / % possible obs: 99.2 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 19.9 % / Biso Wilson estimate: 40.6 Å2 / Rmerge(I) obs: 0.07 / Net I/σ(I): 27.8 |
| Reflection shell | Resolution: 2.5→2.59 Å / Redundancy: 4.1 % / Rmerge(I) obs: 0.413 / Mean I/σ(I) obs: 2.5 / % possible all: 93.5 |
| Reflection | *PLUS Num. measured all: 167936 |
| Reflection shell | *PLUS % possible obs: 93.5 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: N-terminally truncated chicken cystatin (1cew.pdb) converted to a polyalanine chain and limited to those fragments that had been modeled in electron density (residues 86-90 not included) Resolution: 2.5→20 Å / Rfactor Rfree error: 0.008 / Isotropic thermal model: individual isotropic / Cross valid method: free R / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber / Details: maximum likelihood
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: flat model / Bsol: 38.73 Å2 / ksol: 0.33 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→20 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.59 Å / Rfactor Rfree error: 0.04 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 0.9 / Classification: refinement | ||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj





