+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ema | ||||||
|---|---|---|---|---|---|---|---|
| Title | GREEN FLUORESCENT PROTEIN FROM AEQUOREA VICTORIA | ||||||
 Components Components | GREEN FLUORESCENT PROTEIN | ||||||
 Keywords Keywords | FLUORESCENT PROTEIN / BETA-BARREL / AUTOCATALYTIC / FLUOROPHORE / BIOLUMINESCENSE FLUORESCENT PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / MIR/SAD / Resolution: 1.9 Å SYNCHROTRON / MIR/SAD / Resolution: 1.9 Å | ||||||
 Authors Authors | Ormo, M. / Remington, S.J. | ||||||
 Citation Citation |  Journal: Science / Year: 1996 Journal: Science / Year: 1996Title: Crystal structure of the Aequorea victoria green fluorescent protein. Authors: Ormo, M. / Cubitt, A.B. / Kallio, K. / Gross, L.A. / Tsien, R.Y. / Remington, S.J. #1:  Journal: Nature / Year: 1995 Journal: Nature / Year: 1995Title: Improved Green Fluorescence Authors: Heim, R. / Cubitt, A.B. / Tsien, R.Y. | ||||||
| History |
|
- Structure visualization
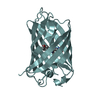
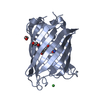
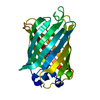
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ema.cif.gz 1ema.cif.gz | 60 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ema.ent.gz pdb1ema.ent.gz | 43.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ema.json.gz 1ema.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/em/1ema https://data.pdbj.org/pub/pdb/validation_reports/em/1ema ftp://data.pdbj.org/pub/pdb/validation_reports/em/1ema ftp://data.pdbj.org/pub/pdb/validation_reports/em/1ema | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 27226.754 Da / Num. of mol.: 1 / Mutation: 65 - 67 REPLACED BY CRO, Q80R Source method: isolated from a genetically manipulated source Details: THE PROTEIN CONTAINS SIX SE-METHIONINES. OF THESE, THE N-TERMINAL MET AND MET 233 ARE NOT PRESENT IN THE ENTRY Source: (gene. exp.)   |
|---|---|
| #2: Water | ChemComp-HOH / |
| Has protein modification | Y |
| Sequence details | THE FLUOROPHORE (CRO) IS GENERATED BY AN AUTOCATALYTIC CYCLIZATION OF THE POLYPEPTIDE BACKBONE ...THE FLUOROPHOR |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.1 Å3/Da / Density % sol: 40.5 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 8.2 Details: 22-26% PEG 4000, 50 MM HEPES PH 8.0-8.4, 50 MM MGCL2, 10 MM 2-MERCAPTOETHANOL, 5-7 MG PROTEIN, pH 8.2 PH range: 8.0-8.4 | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 295 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X4A / Wavelength: 0.979 / Beamline: X4A / Wavelength: 0.979 |
| Detector | Type: FUJI / Detector: IMAGE PLATE / Date: Apr 28, 1996 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Highest resolution: 1.9 Å / Num. obs: 17676 / % possible obs: 84 % / Observed criterion σ(I): 0 / Redundancy: 6.45 % / Biso Wilson estimate: 16.5 Å2 / Rmerge(I) obs: 0.09 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: MIR/SAD / Resolution: 1.9→20 Å / Isotropic thermal model: TNT / σ(F): 0 / Stereochemistry target values: TNT Details: THE FINAL (FO-FC) DENSITY SHOWS LARGE DIFFERENCE FEATURES LOCATED AROUND THE MAIN CHAIN PART OF THE FLUOROPHORE THAT ORIGINATES FROM RESIDUES THR 65 AND GLY 67. THE DIFFERENCE DENSITIES ALSO ...Details: THE FINAL (FO-FC) DENSITY SHOWS LARGE DIFFERENCE FEATURES LOCATED AROUND THE MAIN CHAIN PART OF THE FLUOROPHORE THAT ORIGINATES FROM RESIDUES THR 65 AND GLY 67. THE DIFFERENCE DENSITIES ALSO AFFECT THE ATOMIC-POSITION REFINEMENT OF VAL 68. THE DIFFERENCE FEATURES MIGHT BE EXPLAINED AS A SUBSET (<30%) OF MOLECULES THAT HAS FAILED TO UNDERGO THE COMPLETE FORMATION OF THE FLUOROPHORE. THE LOOP RESIDUES 157 AND 158 ARE DISORDERED. A NUMBER OF SURFACE RESIDUES HAVE TRUNCATED SIDE CHAINS DUE TO WEAK OR NO DENSITY.
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: BABINET SCALING / Bsol: 300 Å2 / ksol: 0.8 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: TNT / Version: 5-F / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.175 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 24.1 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller




















 PDBj
PDBj


