+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2okw | ||||||
|---|---|---|---|---|---|---|---|
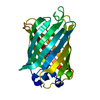
| Title | A non-invasive GFP-based biosensor for mercury ions | ||||||
 Components Components | Green fluorescent protein | ||||||
 Keywords Keywords | LUMINESCENT PROTEIN / mercury / biosensor | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Chapleau, R.R. / Blomberg, R. / Ford, P.C. / Sagermann, M. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 2008 Journal: Protein Sci. / Year: 2008Title: Design of a highly specific and noninvasive biosensor suitable for real-time in vivo imaging of mercury (II) uptake. Authors: Chapleau, R.R. / Blomberg, R. / Ford, P.C. / Sagermann, M. #1:  Journal: Proc.Natl.Acad.Sci.USA / Year: 2004 Journal: Proc.Natl.Acad.Sci.USA / Year: 2004Title: Local complexity of amino acid interactions in a protein core. Authors: Jain, R.K. / Ranganathan, R. #2:  Journal: Science / Year: 1996 Journal: Science / Year: 1996Title: Crystal structure of the Aequorea victoria green fluorescent protein. Authors: Ormo, M. / Cubitt, A.B. / Kallio, K. / Gross, L.A. / Tsien, R.Y. / Remington, S.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2okw.cif.gz 2okw.cif.gz | 293.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2okw.ent.gz pdb2okw.ent.gz | 237.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2okw.json.gz 2okw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ok/2okw https://data.pdbj.org/pub/pdb/validation_reports/ok/2okw ftp://data.pdbj.org/pub/pdb/validation_reports/ok/2okw ftp://data.pdbj.org/pub/pdb/validation_reports/ok/2okw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2okyC  1embS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 26898.365 Da / Num. of mol.: 6 / Mutation: S65T, R80Q, S205C Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | AUTHORS STATE THAT RESIDUE 64 IS A LEUCINE. RESIDUE 65 HAS BEEN MUTATED FROM SER TO THR AND RESIDUE ...AUTHORS STATE THAT RESIDUE 64 IS A LEUCINE. RESIDUE 65 HAS BEEN MUTATED FROM SER TO THR AND RESIDUE 80 IS AN ARG TO GLN MUTATION. RESIDUE 80 IS LISTED AS A GLN IN THE DATABASE REFERENCE BUT IS AN ARG ACCORDING TO ROUWENDAL ET AL., 1997 PLANT MOL. BIOL. 33, 989-999. RESIDUES THR 65, TYR 66, GLY 67 CONSTITUTE | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.6 Å3/Da / Density % sol: 52.75 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 100mM Pipes, 30% PEG 8000, 200mM Na-acetate, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 150 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL11-1 / Wavelength: 0.979454 Å / Beamline: BL11-1 / Wavelength: 0.979454 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Jun 3, 2006 / Details: single SI crystal flat mirror |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979454 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→19.83 Å / Num. all: 156061 / Num. obs: 155659 / % possible obs: 99.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7.16 % / Rmerge(I) obs: 0.073 / Net I/σ(I): 15.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1EMB Resolution: 1.9→19.83 Å / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber Details: First 6 residues and the last c-terminal residues could not be modeled into the density. These residues were therefore omitted in the final model
| |||||||||||||||||||||||||
| Displacement parameters |
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→19.83 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj


