[English] 日本語
 Yorodumi
Yorodumi- PDB-1d4l: HIV-1 PROTEASE COMPLEXED WITH A MACROCYCLIC PEPTIDOMIMETIC INHIBITOR -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1d4l | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIV-1 PROTEASE COMPLEXED WITH A MACROCYCLIC PEPTIDOMIMETIC INHIBITOR | ||||||
 Components Components | HIV-1 PROTEASE | ||||||
 Keywords Keywords | HYDROLASE / HIV / PROTEASE / INHIBITOR / ANTIVIRAL | ||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding Similarity search - Function | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.75 Å X-RAY DIFFRACTION / Resolution: 1.75 Å | ||||||
 Authors Authors | Tyndall, J.D. / Reid, R.C. / Tyssen, D.P. / Jardine, D.K. / Todd, B. / Passmore, M. / March, D.R. / Pattenden, L.K. / Alewood, D. / Hu, S.H. ...Tyndall, J.D. / Reid, R.C. / Tyssen, D.P. / Jardine, D.K. / Todd, B. / Passmore, M. / March, D.R. / Pattenden, L.K. / Alewood, D. / Hu, S.H. / Alewood, P.F. / Birch, C.J. / Martin, J.L. / Fairlie, D.P. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2000 Journal: J.Med.Chem. / Year: 2000Title: Synthesis, stability, antiviral activity, and protease-bound structures of substrate-mimicking constrained macrocyclic inhibitors of HIV-1 protease. Authors: Tyndall, J.D. / Reid, R.C. / Tyssen, D.P. / Jardine, D.K. / Todd, B. / Passmore, M. / March, D.R. / Pattenden, L.K. / Bergman, D.A. / Alewood, D. / Hu, S.H. / Alewood, P.F. / Birch, C.J. / ...Authors: Tyndall, J.D. / Reid, R.C. / Tyssen, D.P. / Jardine, D.K. / Todd, B. / Passmore, M. / March, D.R. / Pattenden, L.K. / Bergman, D.A. / Alewood, D. / Hu, S.H. / Alewood, P.F. / Birch, C.J. / Martin, J.L. / Fairlie, D.P. #1:  Journal: Biochemistry / Year: 1999 Journal: Biochemistry / Year: 1999Title: Molecular Recognition of Macrocyclic Peptidomimetic Inhibitors by HIV-1 Protease. Authors: Martin, J.L. / Begun, J. / Schindeler, A. / Wickramasinghe, W.A. / Alewood, D. / Alewood, P.F. / Bergman, D.A. / Brinkworth, R.I. / Abbenante, G. / March, D. / Reid, R.C. / Fairlie, D.P. #2:  Journal: J.Am.Chem.Soc. / Year: 1996 Journal: J.Am.Chem.Soc. / Year: 1996Title: Substrate Based Cyclic Peptidomimetics Of Phe Ile Val That Inhibit HIV-1 Protease Using a Novel Enzyme Binding Mode. Authors: March, D. / Abbenante, G. / Bergman, D. / Brinkworth, R.I. / Wickramasinghe, W. / Begun, J. / Martin, J.L. / Fairlie, D.P. #3:  Journal: J.Am.Chem.Soc. / Year: 1995 Journal: J.Am.Chem.Soc. / Year: 1995Title: Regioselective Structural and Functional Mimicry Of Peptides: Design Of Hydrolytically Stable Cyclic Peptidomimetic Inhibitors Of HIV-1 Protease. Authors: Abbenante, G. / March, D. / Bergman, D. / Hunt, P.A. / Garnham, B. / Dancer, R.J. / Martin, J.L. / Fairlie, D.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1d4l.cif.gz 1d4l.cif.gz | 52.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1d4l.ent.gz pdb1d4l.ent.gz | 41 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1d4l.json.gz 1d4l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d4/1d4l https://data.pdbj.org/pub/pdb/validation_reports/d4/1d4l ftp://data.pdbj.org/pub/pdb/validation_reports/d4/1d4l ftp://data.pdbj.org/pub/pdb/validation_reports/d4/1d4l | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 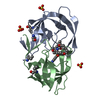
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 10765.687 Da / Num. of mol.: 2 / Mutation: Q7K, L33I, C67(ABA), C95(ABA) / Source method: obtained synthetically Details: SF2 isolate, chemically synthesised protein corresponds to the protease from HIV-1, with 4 mutations per monomer References: UniProt: P03369, HIV-1 retropepsin #2: Chemical | #3: Chemical | ChemComp-PI9 / ( | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.16 Å3/Da / Density % sol: 43.18 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 5.5 Details: ammonium sulfate. acetate buffer, , pH 5.5, VAPOR DIFFUSION, HANGING DROP, temperature 293.0K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Details: inhibitor:protein molar ratio is 10:1 | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.54 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.54 |
| Detector | Type: RIGAKU RAXIS IIC / Detector: IMAGE PLATE / Date: May 8, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→50 Å / Num. all: 62454 / Num. obs: 59019 / % possible obs: 94.5 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 1 / Redundancy: 3.38 % / Biso Wilson estimate: 10.2 Å2 / Rmerge(I) obs: 0.083 / Net I/σ(I): 9.5 |
| Reflection shell | Resolution: 1.75→1.83 Å / Redundancy: 3.35 % / Rmerge(I) obs: 0.302 / Num. unique all: 1621 / % possible all: 84.6 |
| Reflection | *PLUS Num. obs: 18470 / Num. measured all: 62454 |
| Reflection shell | *PLUS % possible obs: 84.6 % / Mean I/σ(I) obs: 2.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.75→8 Å / Rfactor Rfree error: 0.005 / Data cutoff high absF: 10000000 / Data cutoff low absF: 0.001 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 19.5 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.75→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.75→1.86 Å / Rfactor Rfree error: 0.02 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 0 / % reflection Rfree: 9.8 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 19.5 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.32 / % reflection Rfree: 9.3 % / Rfactor Rwork: 0.281 / Rfactor obs: 0.288 |
 Movie
Movie Controller
Controller













 PDBj
PDBj