[English] 日本語
 Yorodumi
Yorodumi- PDB-1a9t: BOVINE PURINE NUCLEOSIDE PHOSPHORYLASE COMPLEXED WITH 9-DEAZAINOS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1a9t | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BOVINE PURINE NUCLEOSIDE PHOSPHORYLASE COMPLEXED WITH 9-DEAZAINOSINE AND PHOSPHATE | |||||||||
 Components Components | PURINE NUCLEOSIDE PHOSPHORYLASE | |||||||||
 Keywords Keywords | TRANSFERASE / GLYCOSYLTRANSFERASE / PENTOSYLTRANSFERASE / PURINE NUCLEOSIDE PHOSPHORYLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationguanosine phosphorylase activity / purine-nucleoside phosphorylase / purine-nucleoside phosphorylase activity / purine ribonucleoside salvage / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | |||||||||
 Authors Authors | Mao, C. / Cook, W.J. / Zhou, M. / Fedorov, A.A. / Almo, S.C. / Ealick, S.E. | |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: Calf spleen purine nucleoside phosphorylase complexed with substrates and substrate analogues. Authors: Mao, C. / Cook, W.J. / Zhou, M. / Federov, A.A. / Almo, S.C. / Ealick, S.E. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1a9t.cif.gz 1a9t.cif.gz | 68.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1a9t.ent.gz pdb1a9t.ent.gz | 50.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1a9t.json.gz 1a9t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/a9/1a9t https://data.pdbj.org/pub/pdb/validation_reports/a9/1a9t ftp://data.pdbj.org/pub/pdb/validation_reports/a9/1a9t ftp://data.pdbj.org/pub/pdb/validation_reports/a9/1a9t | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1a9oC  1a9pC 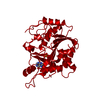 1a9qC  1a9rC  1a9sC  1pbnSC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 31567.992 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P55859, purine-nucleoside phosphorylase |
|---|---|
| #2: Sugar | ChemComp-R1P / |
| #3: Chemical | ChemComp-HPA / |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.23 % | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Details: PROTEIN WAS CRYSTALLIZED FROM 31-35% PEG-400 IN 100 MM HEPES OR TRIS BUFFER, PH 7.8-8.2; 100 MM MGCL2; 1% OCTYL-BETA- D-GLUCOPYRANOSIDE PH range: 7.8-8.2 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop / PH range low: 8.2 / PH range high: 7.8 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 296 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Detector | Type: XUONG-HAMLIN MULTIWIRE / Detector: AREA DETECTOR / Date: Aug 1, 1996 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2→8 Å / Num. obs: 14891 / % possible obs: 80 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1PBN Resolution: 2→8 Å / Cross valid method: THROUGHOUT / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.8 / Classification: refinement X-PLOR / Version: 3.8 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj