[English] 日本語
 Yorodumi
Yorodumi- EMDB-5144: Ab initio reconstruction of a poliovirus-receptor complex via the... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5144 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ab initio reconstruction of a poliovirus-receptor complex via the asymmetric random-model method | |||||||||
 Map data Map data | Poliovirus-receptor complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | random-model method / ab initio reconstruction / poliovirus-receptor complex. | |||||||||
| Biological species |  Human enterovirus C Human enterovirus C | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 30.0 Å | |||||||||
 Authors Authors | Sanz E / Stewart AB / Belnap DM | |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2010 Journal: J Struct Biol / Year: 2010Title: The random-model method enables ab initio 3D reconstruction of asymmetric particles and determination of particle symmetry. Authors: Eduardo Sanz-García / Aaron B Stewart / David M Belnap /  Abstract: Model-based, 3D reconstruction techniques depend on reliable starting models. We present an extension of the random-model method (RMM) that allows the ab initio generation of suitable starting models ...Model-based, 3D reconstruction techniques depend on reliable starting models. We present an extension of the random-model method (RMM) that allows the ab initio generation of suitable starting models directly from un-averaged, experimental images of asymmetric or symmetric particles. Therefore, the asymmetric RMM can also be used to determine point-group symmetry. The procedure is facilitated by the use of (a) variable angular step-sizes during iterative origin and orientation searches, (b) high numbers of particle images, and (c) highly defocused images. The method is inhibited by mixed-handedness orientation assignments and by particles with inconspicuous features. For symmetric particles, symmetric RMMs can overcome these deficiencies. #1:  Journal: PROC.NAT.ACAD.SCI.USA / Year: 2000 Journal: PROC.NAT.ACAD.SCI.USA / Year: 2000Title: Three-dimensional structure of poliovirus receptor bound to poliovirus Authors: Belnap DM / McDermott BM Jr / Filman DJ / Cheng N / Trus BL / Zuccola HJ / Racaniello VR / Hogle JM / Steven AC | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5144.map.gz emd_5144.map.gz | 44.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5144-v30.xml emd-5144-v30.xml emd-5144.xml emd-5144.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| Images |  400_5144.gif 400_5144.gif 80_5144.gif 80_5144.gif emd_5144_1.tif emd_5144_1.tif | 63.5 KB 5.7 KB 489.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5144 http://ftp.pdbj.org/pub/emdb/structures/EMD-5144 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5144 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5144 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5144.map.gz / Format: CCP4 / Size: 88.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5144.map.gz / Format: CCP4 / Size: 88.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Poliovirus-receptor complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.84 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
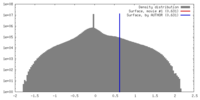
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Poliovirus-receptor complex
| Entire | Name: Poliovirus-receptor complex |
|---|---|
| Components |
|
-Supramolecule #1000: Poliovirus-receptor complex
| Supramolecule | Name: Poliovirus-receptor complex / type: sample / ID: 1000 Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to ...Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to poliovirus, Proc. Natl. Acad. Sci. USA 97 (2000) 73-78. Oligomeric state: 1 virus binds 60 receptors / Number unique components: 2 |
|---|
-Supramolecule #1: Human enterovirus C
| Supramolecule | Name: Human enterovirus C / type: virus / ID: 1 / Name.synonym: Poliovirus Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to ...Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to poliovirus, Proc. Natl. Acad. Sci. USA 97 (2000) 73-78. NCBI-ID: 138950 / Sci species name: Human enterovirus C / Database: NCBI / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No / Syn species name: Poliovirus |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
| Virus shell | Shell ID: 1 / Name: VP1 VP2 VP3 / Diameter: 242 Å / T number (triangulation number): 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Vitrification | Cryogen name: ETHANE / Instrument: OTHER |
|---|
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Details | D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to poliovirus, Proc. Natl. Acad. Sci. USA 97 (2000) 73-78. |
| Image recording | Category: CCD / Film or detector model: GENERIC FILM Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to ...Details: D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to poliovirus, Proc. Natl. Acad. Sci. USA 97 (2000) 73-78. |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | D.M. Belnap, B.M. McDermott, Jr., D.J. Filman, N. Cheng, B.L. Trus, H.J. Zuccola, V.R. Racaniello, J.M. Hogle, A.C. Steven, Three-dimensional structure of poliovirus receptor bound to poliovirus, Proc. Natl. Acad. Sci. USA 97 (2000) 73-78. |
|---|---|
| CTF correction | Details: Phase-flipped |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 30.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: PFT3DR, Bsoft Details: Random-model method. Angular step-size was initially set to 20 deg. in the first iteration and gradually decreased by 0.19 deg. in each successive iteration, until a lower limit of 1 deg. was reached. Number images used: 5626 |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)