+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-2746 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Conformational Snapshots of Inducible Nitric Oxide Synthase (iNOS) | |||||||||
 マップデータ マップデータ | Single-particle reconstruction of iNOS: Group III, Conformation i | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | heterogeneity / random conical tilt / nitric oxide synthase / calmodulin / heme / electron transfer / flavin | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Nitric oxide stimulates guanylate cyclase / ROS and RNS production in phagocytes / peptidyl-cysteine S-nitrosylation / Peroxisomal protein import / prostaglandin secretion / tetrahydrobiopterin binding / arginine binding / superoxide metabolic process / regulation of cytokine production involved in inflammatory response / cortical cytoskeleton ...Nitric oxide stimulates guanylate cyclase / ROS and RNS production in phagocytes / peptidyl-cysteine S-nitrosylation / Peroxisomal protein import / prostaglandin secretion / tetrahydrobiopterin binding / arginine binding / superoxide metabolic process / regulation of cytokine production involved in inflammatory response / cortical cytoskeleton / cellular response to cytokine stimulus / Fc-gamma receptor signaling pathway involved in phagocytosis / nitric-oxide synthase (NADPH) / nitric-oxide synthase activity / L-arginine catabolic process / nitric oxide biosynthetic process / regulation of insulin secretion / positive regulation of interleukin-8 production / response to bacterium / circadian rhythm / negative regulation of protein catabolic process / cellular response to type II interferon / positive regulation of interleukin-6 production / cellular response to xenobiotic stimulus / peroxisome / FMN binding / flavin adenine dinucleotide binding / NADP binding / regulation of cell population proliferation / cellular response to lipopolysaccharide / response to lipopolysaccharide / calmodulin binding / response to hypoxia / defense response to bacterium / iron ion binding / inflammatory response / negative regulation of gene expression / heme binding / perinuclear region of cytoplasm / protein homodimerization activity / metal ion binding / cytoplasm / cytosol 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
| 手法 | 単粒子再構成法 / ネガティブ染色法 / 解像度: 69.0 Å | |||||||||
 データ登録者 データ登録者 | Campbell MG / Smith BC / Potter CS / Carragher B / Marletta MA | |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2014 ジャーナル: Proc Natl Acad Sci U S A / 年: 2014タイトル: Molecular architecture of mammalian nitric oxide synthases. 著者: Melody G Campbell / Brian C Smith / Clinton S Potter / Bridget Carragher / Michael A Marletta /  要旨: NOSs are homodimeric multidomain enzymes responsible for producing NO. In mammals, NO acts as an intercellular messenger in a variety of signaling reactions, as well as a cytotoxin in the innate ...NOSs are homodimeric multidomain enzymes responsible for producing NO. In mammals, NO acts as an intercellular messenger in a variety of signaling reactions, as well as a cytotoxin in the innate immune response. Mammals possess three NOS isoforms--inducible, endothelial, and neuronal NOS--that are composed of an N-terminal oxidase domain and a C-terminal reductase domain. Calmodulin (CaM) activates NO synthesis by binding to the helical region connecting these two domains. Although crystal structures of isolated domains have been reported, no structure is available for full-length NOS. We used high-throughput single-particle EM to obtain the structures and higher-order domain organization of all three NOS holoenzymes. The structures of inducible, endothelial, and neuronal NOS with and without CaM bound are similar, consisting of a dimerized oxidase domain flanked by two separated reductase domains. NOS isoforms adopt many conformations enabled by three flexible linkers. These conformations represent snapshots of the continuous electron transfer pathway from the reductase domain to the oxidase domain, which reveal that only a single reductase domain participates in electron transfer at a time, and that CaM activates NOS by constraining rotational motions and by directly binding to the oxidase domain. Direct visualization of these large conformational changes induced during electron transfer provides significant insight into the molecular underpinnings governing NO formation. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_2746.map.gz emd_2746.map.gz | 57.4 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-2746-v30.xml emd-2746-v30.xml emd-2746.xml emd-2746.xml | 10 KB 10 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_2746.png emd_2746.png | 26.7 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2746 http://ftp.pdbj.org/pub/emdb/structures/EMD-2746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2746 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_2746_validation.pdf.gz emd_2746_validation.pdf.gz | 191.3 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_2746_full_validation.pdf.gz emd_2746_full_validation.pdf.gz | 190.4 KB | 表示 | |
| XML形式データ |  emd_2746_validation.xml.gz emd_2746_validation.xml.gz | 7 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2746 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2746 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2746 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2746 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  2718C  2719C  2720C  2721C  2722C  2723C  2724C  2725C  2726C  2727C  2728C  2729C  2730C 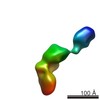 2731C  2732C  2733C  2734C  2735C  2736C  2737C  2738C  2739C  2740C  2741C  2742C  2743C  2744C  2745C  2747C  2748C  2749C C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_2746.map.gz / 形式: CCP4 / 大きさ: 162.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_2746.map.gz / 形式: CCP4 / 大きさ: 162.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Single-particle reconstruction of iNOS: Group III, Conformation i | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.36 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Murine Inducible Nitric Oxide Synthase
| 全体 | 名称: Murine Inducible Nitric Oxide Synthase |
|---|---|
| 要素 |
|
-超分子 #1000: Murine Inducible Nitric Oxide Synthase
| 超分子 | 名称: Murine Inducible Nitric Oxide Synthase / タイプ: sample / ID: 1000 / 詳細: Sample is highly flexible / 集合状態: Homodimer / Number unique components: 1 |
|---|---|
| 分子量 | 理論値: 260 KDa |
-分子 #1: Inducible Nitric Oxide Synthase
| 分子 | 名称: Inducible Nitric Oxide Synthase / タイプ: protein_or_peptide / ID: 1 / Name.synonym: iNOS / コピー数: 1 / 集合状態: Homodimer / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 260 KDa |
| 組換発現 | 生物種:  |
| 配列 | UniProtKB: Nitric oxide synthase, inducible GO: nitric oxide biosynthetic process, FMN binding, NADP binding, calmodulin binding, flavin adenine dinucleotide binding, heme binding, iron ion binding, nitric-oxide synthase activity InterPro: Ser/Thr protein kinase, TGFB receptor |
-実験情報
-構造解析
| 手法 | ネガティブ染色法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 / 詳細: 50 mM TEA pH 7.5, 150 mM NaCl, and 5 mM DTT |
|---|---|
| 染色 | タイプ: NEGATIVE 詳細: 3 microliters of sample were applied to grid. The specimen was stained twice with 2% uranyl formate, then allowed to air-dry. |
| グリッド | 詳細: Glow discharged C-flat grid with 2-micron-diameter holes overlaid by thin 1.5 nm continuous carbon |
| 凍結 | 凍結剤: NONE / 装置: OTHER |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| 日付 | 2013年3月6日 |
| 撮影 | カテゴリ: CCD フィルム・検出器のモデル: TVIPS TEMCAM-F416 (4k x 4k) 実像数: 2226 / 平均電子線量: 37 e/Å2 詳細: Each area is imaged twice: once at 55 degrees and again with no tilt. |
| Tilt angle min | 0 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 114705 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2 mm / 最大 デフォーカス(公称値): 2.5 µm / 最小 デフォーカス(公称値): 1.0 µm / 倍率(公称値): 62000 |
| 試料ステージ | 試料ホルダーモデル: SIDE ENTRY, EUCENTRIC / Tilt angle max: 55 |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | See publication. |
|---|---|
| CTF補正 | 詳細: Each Image |
| 最終 再構成 | 想定した対称性 - 点群: C1 (非対称) / アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 69.0 Å / 解像度の算出法: OTHER / ソフトウェア - 名称: Appion, Spider / 詳細: See publication. / 使用した粒子像数: 248 |
| 最終 2次元分類 | クラス数: 1 |
 ムービー
ムービー コントローラー
コントローラー












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















