+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11790 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of F-actin stabilized by trans-optoJASP-8 | |||||||||
 Map data Map data | Sharpened map of the full filament filtered to local resolution (Bfactor optimised for optoJASP) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cytoskeleton / jasplakinolide / azobenzene photoswitch / stabilized-actin filament / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament ...cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / skeletal muscle fiber development / stress fiber / titin binding / actin filament polymerization / actin filament / filopodium / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding / lamellipodium / cell body / protein domain specific binding / hydrolase activity / calcium ion binding / positive regulation of gene expression / magnesium ion binding / ATP binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
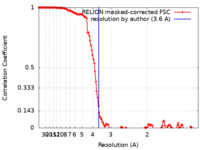
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Pospich S / Raunser S | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Angew Chem Int Ed Engl / Year: 2021 Journal: Angew Chem Int Ed Engl / Year: 2021Title: Cryo-EM Resolves Molecular Recognition Of An Optojasp Photoswitch Bound To Actin Filaments In Both Switch States. Authors: Sabrina Pospich / Florian Küllmer / Veselin Nasufović / Johanna Funk / Alexander Belyy / Peter Bieling / Hans-Dieter Arndt / Stefan Raunser /  Abstract: Actin is essential for key processes in all eukaryotic cells. Cellpermeable optojasps provide spatiotemporal control of the actin cytoskeleton, confining toxicity and potentially rendering F-actin ...Actin is essential for key processes in all eukaryotic cells. Cellpermeable optojasps provide spatiotemporal control of the actin cytoskeleton, confining toxicity and potentially rendering F-actin druggable by photopharmacology. Here, we report cryo electron microscopy (cryo-EM) structures of both isomeric states of one optojasp bound to actin filaments. The high-resolution structures reveal for the first time the pronounced effects of photoswitching a functionalized azobenzene. By characterizing the optojasp binding site and identifying conformational changes within F-actin that depend on the optojasp isomeric state, we refine determinants for the design of functional F-actin photoswitches. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11790.map.gz emd_11790.map.gz | 11.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11790-v30.xml emd-11790-v30.xml emd-11790.xml emd-11790.xml | 31.5 KB 31.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11790_fsc.xml emd_11790_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_11790.png emd_11790.png | 103.8 KB | ||
| Masks |  emd_11790_msk_1.map emd_11790_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-11790.cif.gz emd-11790.cif.gz | 8 KB | ||
| Others |  emd_11790_additional_1.map.gz emd_11790_additional_1.map.gz emd_11790_additional_2.map.gz emd_11790_additional_2.map.gz emd_11790_half_map_1.map.gz emd_11790_half_map_1.map.gz emd_11790_half_map_2.map.gz emd_11790_half_map_2.map.gz | 10.7 MB 12.6 MB 170.3 MB 170.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11790 http://ftp.pdbj.org/pub/emdb/structures/EMD-11790 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11790 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11790 | HTTPS FTP |
-Related structure data
| Related structure data |  7ahqMC  7ahnC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11790.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11790.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of the full filament filtered to local resolution (Bfactor optimised for optoJASP) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.68 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11790_msk_1.map emd_11790_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map of the central 120A of the...
| File | emd_11790_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of the central 120A of the filament filtered to nominal resolution (Bfactor optimised for optoJASP) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map of the central 120A of thel...
| File | emd_11790_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of the central 120A of thel filament filtered to nominal resolution (automatic Bfactor) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of the full filament
| File | emd_11790_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the full filament | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of the full filament
| File | emd_11790_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the full filament | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Filamentous alpha actin stabilized by trans-optoJASP-8 in complex...
| Entire | Name: Filamentous alpha actin stabilized by trans-optoJASP-8 in complex with ADP-Pi |
|---|---|
| Components |
|
-Supramolecule #1: Filamentous alpha actin stabilized by trans-optoJASP-8 in complex...
| Supramolecule | Name: Filamentous alpha actin stabilized by trans-optoJASP-8 in complex with ADP-Pi type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.109973 KDa |
| Sequence | String: MCDEDETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY ...String: MCDEDETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY EGYALPHAIM RLDLAGRDLT DYLMKILTER GYSFVTTAER EIVRDIKEKL CYVALDFENE MATAASSS S LEKSYELPDG QVITIGNERF RCPETLFQPS FIGMESAGIH ETTYNSIMKC DIDIRKDLYA NNVMSGGTTM YPGIADRMQ KEITALAPST MKIKIIAPPE RKYSVWIGGS ILASLSTFQQ MWITKQEYDE AGPSIVHRKC F UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 5 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 3 / Number of copies: 5 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 5 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: ~{N}-[4-[(4~{R},7~{R},10~{S},13~{S},15~{E},19~{S})-4-(4-hydroxyph...
| Macromolecule | Name: ~{N}-[4-[(4~{R},7~{R},10~{S},13~{S},15~{E},19~{S})-4-(4-hydroxyphenyl)-7-(1~{H}-indol-3-ylmethyl)-8,13,15,19-tetramethyl-2,6,9,12-tetrakis(oxidanylidene)-1-oxa-5,8,11-triazacyclononadec-15-en- ...Name: ~{N}-[4-[(4~{R},7~{R},10~{S},13~{S},15~{E},19~{S})-4-(4-hydroxyphenyl)-7-(1~{H}-indol-3-ylmethyl)-8,13,15,19-tetramethyl-2,6,9,12-tetrakis(oxidanylidene)-1-oxa-5,8,11-triazacyclononadec-15-en-10-yl]butyl]-~{N}'-[5-methoxy-2-[(~{Z})-(3,4,5-trimethoxyphenyl)diazenyl]phenyl]butanediamide type: ligand / ID: 5 / Number of copies: 5 / Formula: RLZ |
|---|---|
| Molecular weight | Theoretical: 1.073239 KDa |
| Chemical component information | 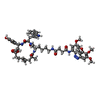 ChemComp-RLZ: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 5 mM Tris pH 7.5, 2 mM NaN3, 1 mM DTT, 100 mM KCl and 2 mM MgCl2, 0.7 %(v/v) DMSO, 0.02 %(v/w) Tween 20 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 286 K / Instrument: FEI VITROBOT MARK III Details: 1.5 mul sample, automatic blotting for 7-7.5s, blot force -25, drain time 1s.. | ||||||||||||||||||||||||
| Details | Rise 27.5 A, Twist -166.6 degrees |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: Cs-corrected |
| Details | Cs-corrected |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Number real images: 7178 / Average exposure time: 1.5 sec. / Average electron dose: 92.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 0.0 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: C / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | An initial model of trans-optoJASP-8 was generated using elBow within Phenix inputting the SMILES string. |
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-7ahq: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)