[日本語] English
 万見
万見- EMDB-23939: Cryo-EM structure of the human SSU processome, state pre-A1 - raw maps -
+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-23939 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of the human SSU processome, state pre-A1 - raw maps | |||||||||
 マップデータ マップデータ | Main map | |||||||||
 試料 試料 |
| |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報mRNA N-acetyltransferase activity / U6 snRNA 2'-O-ribose methyltransferase activity / oocyte growth / nucleologenesis / snoRNA localization / leucine zipper domain binding / granular component / tRNA wobble cytosine modification / U4atac snRNP / tRNA cytidine N4-acetyltransferase activity ...mRNA N-acetyltransferase activity / U6 snRNA 2'-O-ribose methyltransferase activity / oocyte growth / nucleologenesis / snoRNA localization / leucine zipper domain binding / granular component / tRNA wobble cytosine modification / U4atac snRNP / tRNA cytidine N4-acetyltransferase activity / rRNA acetylation involved in maturation of SSU-rRNA / 18S rRNA cytidine N-acetyltransferase activity / regulation of stem cell population maintenance / tRNA acetylation / U4atac snRNA binding / CURI complex / UTP-C complex / negative regulation of amyloid precursor protein biosynthetic process / pre-snoRNP complex / t-UTP complex / Pwp2p-containing subcomplex of 90S preribosome / Mpp10 complex / rRNA (pseudouridine) methyltransferase activity / box C/D sno(s)RNA binding / rRNA modification / histone H2AQ104 methyltransferase activity / preribosome / dense fibrillar component / histone methyltransferase binding / box C/D sno(s)RNA 3'-end processing / rRNA methyltransferase activity / endonucleolytic cleavage in 5'-ETS of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / endonucleolytic cleavage of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / regulation of centrosome duplication / regulation of transcription elongation by RNA polymerase II / endonucleolytic cleavage to generate mature 5'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / N-acetyltransferase activity / cilium disassembly / box C/D methylation guide snoRNP complex / embryonic cleavage / positive regulation of rRNA processing / tRNA export from nucleus / RNA splicing, via transesterification reactions / transcription elongation factor activity / rRNA primary transcript binding / blastocyst formation / sno(s)RNA-containing ribonucleoprotein complex / rRNA base methylation / SUMOylation of RNA binding proteins / U4 snRNA binding / telomerase holoenzyme complex / U2-type precatalytic spliceosome / protein localization to nucleolus / spindle assembly involved in female meiosis / epigenetic programming in the zygotic pronuclei / negative regulation of RNA splicing / rRNA methylation / box C/D snoRNP assembly / neural precursor cell proliferation / neural crest cell differentiation / U3 snoRNA binding / negative regulation of bicellular tight junction assembly / rRNA modification in the nucleus and cytosol / erythrocyte homeostasis / Formation of the ternary complex, and subsequently, the 43S complex / cytoplasmic side of rough endoplasmic reticulum membrane / protein acetylation / snoRNA binding / precatalytic spliceosome / preribosome, small subunit precursor / NRAGE signals death through JNK / negative regulation of ubiquitin protein ligase activity / Cul4-RING E3 ubiquitin ligase complex / Ribosomal scanning and start codon recognition / rRNA metabolic process / Translation initiation complex formation / Association of TriC/CCT with target proteins during biosynthesis / positive regulation of transcription by RNA polymerase I / negative regulation of telomere maintenance via telomerase / RNA polymerase II complex binding / decidualization / TFIID-class transcription factor complex binding / TOR signaling / Protein hydroxylation / SARS-CoV-1 modulates host translation machinery / cellular response to ethanol / mTORC1-mediated signalling / negative regulation of apoptotic signaling pathway / Peptide chain elongation / Selenocysteine synthesis / Formation of a pool of free 40S subunits / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / Eukaryotic Translation Termination / blastocyst development / chromosome, centromeric region / ubiquitin ligase inhibitor activity / SRP-dependent cotranslational protein targeting to membrane / Response of EIF2AK4 (GCN2) to amino acid deficiency / negative regulation of ubiquitin-dependent protein catabolic process / Viral mRNA Translation 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
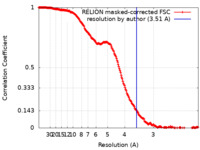
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.51 Å | |||||||||
 データ登録者 データ登録者 | Vanden Broeck A / Singh S / Klinge S | |||||||||
| 資金援助 |  米国, 2件 米国, 2件
| |||||||||
 引用 引用 |  ジャーナル: Science / 年: 2021 ジャーナル: Science / 年: 2021タイトル: Nucleolar maturation of the human small subunit processome. 著者: Sameer Singh / Arnaud Vanden Broeck / Linamarie Miller / Malik Chaker-Margot / Sebastian Klinge /  要旨: The human small subunit processome mediates early maturation of the small ribosomal subunit by coupling RNA folding to subsequent RNA cleavage and processing steps. We report the high-resolution ...The human small subunit processome mediates early maturation of the small ribosomal subunit by coupling RNA folding to subsequent RNA cleavage and processing steps. We report the high-resolution cryo–electron microscopy structures of maturing human small subunit (SSU) processomes at resolutions of 2.7 to 3.9 angstroms. These structures reveal the molecular mechanisms that enable crucial progressions during SSU processome maturation. RNA folding states within these particles are communicated to and coordinated with key enzymes that drive irreversible steps such as targeted exosome-mediated RNA degradation, protein-guided site-specific endonucleolytic RNA cleavage, and tightly controlled RNA unwinding. These conserved mechanisms highlight the SSU processome’s impressive structural plasticity, which endows this 4.5-megadalton nucleolar assembly with the distinctive ability to mature the small ribosomal subunit from within. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_23939.map.gz emd_23939.map.gz | 50.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-23939-v30.xml emd-23939-v30.xml emd-23939.xml emd-23939.xml | 18.1 KB 18.1 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_23939_fsc.xml emd_23939_fsc.xml | 19.8 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_23939.png emd_23939.png | 147.7 KB | ||
| マスクデータ |  emd_23939_msk_1.map emd_23939_msk_1.map | 669.9 MB |  マスクマップ マスクマップ | |
| その他 |  emd_23939_additional_1.map.gz emd_23939_additional_1.map.gz emd_23939_half_map_1.map.gz emd_23939_half_map_1.map.gz emd_23939_half_map_2.map.gz emd_23939_half_map_2.map.gz | 625.2 MB 541.8 MB 541.9 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23939 http://ftp.pdbj.org/pub/emdb/structures/EMD-23939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23939 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_23939_validation.pdf.gz emd_23939_validation.pdf.gz | 509.6 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_23939_full_validation.pdf.gz emd_23939_full_validation.pdf.gz | 509.1 KB | 表示 | |
| XML形式データ |  emd_23939_validation.xml.gz emd_23939_validation.xml.gz | 27.6 KB | 表示 | |
| CIF形式データ |  emd_23939_validation.cif.gz emd_23939_validation.cif.gz | 36.7 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23939 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23939 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7mq8C  7mq9C  7mqaC  7mqjC C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| 電子顕微鏡画像生データ |  EMPIAR-10781 (タイトル: Nucleolar maturation of the human small subunit processome EMPIAR-10781 (タイトル: Nucleolar maturation of the human small subunit processomeData size: 74.6 TB Data #1: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 1 [micrographs - multiframe] Data #2: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 2 [micrographs - multiframe] Data #3: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 3 [micrographs - multiframe] Data #4: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 4 [micrographs - multiframe] Data #5: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 5 [micrographs - multiframe] Data #6: Unaligned multi-frame micrograph movies of human SSU processomes - Dataset 6 [micrographs - multiframe] Data #7: Aligned and averaged micrographs of human SSU processomes - Dataset 1 [micrographs - single frame] Data #8: Aligned and averaged micrographs of human SSU processomes - Dataset 2 [micrographs - single frame] Data #9: Aligned and averaged micrographs of human SSU processomes - Dataset 3 [micrographs - single frame] Data #10: Aligned and averaged micrographs of human SSU processomes - Dataset 4 [micrographs - single frame] Data #11: Aligned and averaged micrographs of human SSU processomes - Dataset 5 [micrographs - single frame] Data #12: Aligned and averaged micrographs of human SSU processomes - Dataset 6 [micrographs - single frame]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_23939.map.gz / 形式: CCP4 / 大きさ: 669.9 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_23939.map.gz / 形式: CCP4 / 大きさ: 669.9 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Main map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-マスク #1
| ファイル |  emd_23939_msk_1.map emd_23939_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-追加マップ: #1
| ファイル | emd_23939_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: Half-Map1
| ファイル | emd_23939_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half-Map1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: Half-Map2
| ファイル | emd_23939_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half-Map2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Human SSU processome, state pre-A1
| 全体 | 名称: Human SSU processome, state pre-A1 |
|---|---|
| 要素 |
|
-超分子 #1: Human SSU processome, state pre-A1
| 超分子 | 名称: Human SSU processome, state pre-A1 / タイプ: complex / ID: 1 / 親要素: 0 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 5 MDa |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.6 |
|---|---|
| グリッド | モデル: Quantifoil R2/2 / 材質: GOLD / メッシュ: 400 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 支持フィルム - Film thickness: 3.0 nm / 前処理 - タイプ: GLOW DISCHARGE |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 90 % / チャンバー内温度: 283 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 実像数: 84904 / 平均電子線量: 58.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 0.01 mm / 最大 デフォーカス(公称値): 2.7 µm 最小 デフォーカス(公称値): 0.7000000000000001 µm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | 空間: REAL |
|---|
 ムービー
ムービー コントローラー
コントローラー







































































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)