[English] 日本語
 Yorodumi
Yorodumi- EMDB-20220: CRYO-EM STRUCTURE OF PHOSPHORYLATED AP-2 (mu E302K) BOUND TO NECA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20220 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CRYO-EM STRUCTURE OF PHOSPHORYLATED AP-2 (mu E302K) BOUND TO NECAP IN THE PRESENCE OF SS DNA | |||||||||
 Map data Map data | cryo-EM structure of phosphorylated AP2 bound to NECAP in the presence of DNA; sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AP2 / NECAP2 PROTEIN / CYTOPLASMIC VESICLE / ENDOCYTOSIS / LIPID-BINDING / ADAPTOR / MEMBRANE / TRANSPORT / PHOSPHORYLATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationFormation of annular gap junctions / Gap junction degradation / clathrin vesicle coat / LDL clearance / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / WNT5A-dependent internalization of FZD4 / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / LDL clearance / Retrograde neurotrophin signalling / Retrograde neurotrophin signalling ...Formation of annular gap junctions / Gap junction degradation / clathrin vesicle coat / LDL clearance / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / WNT5A-dependent internalization of FZD4 / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / LDL clearance / Retrograde neurotrophin signalling / Retrograde neurotrophin signalling / VLDLR internalisation and degradation / Trafficking of GluR2-containing AMPA receptors / WNT5A-dependent internalization of FZD4 / clathrin adaptor complex / VLDLR internalisation and degradation / cardiac septum development / Recycling pathway of L1 / extrinsic component of presynaptic endocytic zone membrane / MHC class II antigen presentation / regulation of vesicle size / postsynaptic endocytic zone / AP-2 adaptor complex / postsynaptic neurotransmitter receptor internalization / Cargo recognition for clathrin-mediated endocytosis / Recycling pathway of L1 / membrane coat / Clathrin-mediated endocytosis / clathrin coat assembly / positive regulation of synaptic vesicle endocytosis / Cargo recognition for clathrin-mediated endocytosis / clathrin-cargo adaptor activity / Clathrin-mediated endocytosis / vesicle budding from membrane / clathrin-dependent endocytosis / MHC class II antigen presentation / signal sequence binding / positive regulation of protein localization to membrane / coronary vasculature development / neurotransmitter secretion / regulation of hematopoietic stem cell differentiation / ventricular septum development / aorta development / low-density lipoprotein particle receptor binding / clathrin binding / Trafficking of GluR2-containing AMPA receptors / positive regulation of receptor internalization / positive regulation of endocytosis / synaptic vesicle endocytosis / negative regulation of protein localization to plasma membrane / vesicle-mediated transport / Neutrophil degranulation / secretory granule / intracellular protein transport / kidney development / cytoplasmic side of plasma membrane / kinase binding / endocytosis / disordered domain specific binding / synaptic vesicle / protein transport / presynapse / heart development / protein-containing complex assembly / cytoplasmic vesicle / transmembrane transporter binding / postsynapse / protein domain specific binding / intracellular membrane-bounded organelle / synapse / lipid binding / protein kinase binding / protein-containing complex binding / glutamatergic synapse / mitochondrion / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
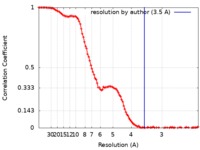
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Partlow EA / Baker RW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: A structural mechanism for phosphorylation-dependent inactivation of the AP2 complex. Authors: Edward A Partlow / Richard W Baker / Gwendolyn M Beacham / Joshua S Chappie / Andres E Leschziner / Gunther Hollopeter /  Abstract: Endocytosis of transmembrane proteins is orchestrated by the AP2 clathrin adaptor complex. AP2 dwells in a closed, inactive state in the cytosol, but adopts an open, active conformation on the plasma ...Endocytosis of transmembrane proteins is orchestrated by the AP2 clathrin adaptor complex. AP2 dwells in a closed, inactive state in the cytosol, but adopts an open, active conformation on the plasma membrane. Membrane-activated complexes are also phosphorylated, but the significance of this mark is debated. We recently proposed that NECAP negatively regulates AP2 by binding open and phosphorylated complexes (Beacham et al., 2018). Here, we report high-resolution cryo-EM structures of NECAP bound to phosphorylated AP2. The site of AP2 phosphorylation is directly coordinated by residues of the NECAP PHear domain that are predicted from genetic screens in . Using membrane mimetics to generate conformationally open AP2, we find that a second domain of NECAP binds these complexes and cryo-EM reveals both domains of NECAP engaging closed, inactive AP2. Assays in vitro and in vivo confirm these domains cooperate to inactivate AP2. We propose that phosphorylation marks adaptors for inactivation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20220.map.gz emd_20220.map.gz | 95.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20220-v30.xml emd-20220-v30.xml emd-20220.xml emd-20220.xml | 26 KB 26 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20220_fsc.xml emd_20220_fsc.xml | 12.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_20220.png emd_20220.png | 56 KB | ||
| Filedesc metadata |  emd-20220.cif.gz emd-20220.cif.gz | 7.7 KB | ||
| Others |  emd_20220_additional.map.gz emd_20220_additional.map.gz emd_20220_half_map_1.map.gz emd_20220_half_map_1.map.gz emd_20220_half_map_2.map.gz emd_20220_half_map_2.map.gz | 51.3 MB 95.4 MB 95.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20220 http://ftp.pdbj.org/pub/emdb/structures/EMD-20220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20220 | HTTPS FTP |
-Related structure data
| Related structure data |  6oxlMC  6owoC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20220.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20220.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM structure of phosphorylated AP2 bound to NECAP in the presence of DNA; sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: cryo-EM structure of phosphorylated AP2 (E302K) bound to...
| File | emd_20220_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM structure of phosphorylated AP2 (E302K) bound to NECAP in the presence of DNA; unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: cryo-EM structure of phosphorylated AP2 (E302K) bound to...
| File | emd_20220_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM structure of phosphorylated AP2 (E302K) bound to NECAP in the presence of DNA; unfiltered half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: cryo-EM structure of phosphorylated AP2 (E302K) bound to...
| File | emd_20220_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM structure of phosphorylated AP2 (E302K) bound to NECAP in the presence of DNA; unfiltered half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Phosphorylated AP2-NECAP (mu E302K) co-complex in the presence of...
| Entire | Name: Phosphorylated AP2-NECAP (mu E302K) co-complex in the presence of ss DNA |
|---|---|
| Components |
|
-Supramolecule #1: Phosphorylated AP2-NECAP (mu E302K) co-complex in the presence of...
| Supramolecule | Name: Phosphorylated AP2-NECAP (mu E302K) co-complex in the presence of ss DNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: AP-2 complex subunit alpha-2
| Macromolecule | Name: AP-2 complex subunit alpha-2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 69.656297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPAVSKGEGM RGLAVFISDI RNCKSKEAEI KRINKELANI RSKFKGDKAL DGYSKKKYVC KLLFIFLLGH DIDFGHMEAV NLLSSNRYT EKQIGYLFIS VLVNSNSELI RLINNAIKND LASRNPTFMG LALHCIANVG SREMAEAFAG EIPKILVAGD T MDSVKQSA ...String: MPAVSKGEGM RGLAVFISDI RNCKSKEAEI KRINKELANI RSKFKGDKAL DGYSKKKYVC KLLFIFLLGH DIDFGHMEAV NLLSSNRYT EKQIGYLFIS VLVNSNSELI RLINNAIKND LASRNPTFMG LALHCIANVG SREMAEAFAG EIPKILVAGD T MDSVKQSA ALCLLRLYRT SPDLVPMGDW TSRVVHLLND QHLGVVTAAT SLITTLAQKN PEEFKTSVSL AVSRLSRIVT SA STDLQDY TYYFVPAPWL SVKLLRLLQC YPPPEDPAVR GRLTECLETI LNKAQEPPKS KKVQHSNAKN AVLFEAISLI IHH DSEPNL LVRACNQLGQ FLQHRETNLR YLALESMCTL ASSEFSHEAV KTHIETVINA LKTERDVSVR QRAVDLLYAM CDRS NAQQI VAEMLSYLET ADYSIREEIV LKVAILAEKY AVDYTWYVDT ILNLIRIAGD YVSEEVWYRV IQIVINRDDV QGYAA KTVF EALQAPACHE NLVKVGGYIL GEFGNLIAGD PRSSPLIQFN LLHSKFHLCS VPTRALLLST YIKFVNLFPE VKATIQ DVL RSDSQLKNAD VELQQRAVEY LRLSTVASTD ILATVLEEMP PFPERESSIL AKLKKKKG |
-Macromolecule #2: AP-2 complex subunit beta
| Macromolecule | Name: AP-2 complex subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 66.953195 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTDSKYFTTN KKGEIFELKA ELNNEKKEKR KEAVKKVIAA MTVGKDVSSL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN SFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQMVEDQ G FLDSLRDL ...String: MTDSKYFTTN KKGEIFELKA ELNNEKKEKR KEAVKKVIAA MTVGKDVSSL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN SFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQMVEDQ G FLDSLRDL IADSNPMVVA NAVAALSEIS ESHPNSNLLD LNPQNINKLL TALNECTEWG QIFILDCLSN YNPKDDREAQ SI CERVTPR LSHANSAVVL SAVKVLMKFL ELLPKDSDYY NMLLKKLAPP LVTLLSGEPE VQYVALRNIN LIVQKRPEIL KQE IKVFFV KYNDPIYVKL EKLDIMIRLA SQANIAQVLA ELKEYATEVD VDFVRKAVRA IGRCAIKVEQ SAERCVSTLL DLIQ TKVNY VVQEAIVVIR DIFRKYPNKY ESIIATLCEN LDSLDEPDAR AAMIWIVGEY AERIDNADEL LESFLEGFHD ESTQV QLTL LTAIVKLFLK KPSETQELVQ QVLSLATQDS DNPDLRDRGY IYWRLLSTDP VTAKEVVLSE KPLISEETDL IEPTLL DEL ICHIGSLASV YHKPPNAFVE GSHGIHRK UniProtKB: AP-2 complex subunit beta |
-Macromolecule #3: AP-2 complex subunit mu
| Macromolecule | Name: AP-2 complex subunit mu / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.806688 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIGGLFIYNH KGEVLISRVY RDDIGRNAVD AFRVNVIHAR QQVRSPVTNI ARTSFFHVKR SNIWLAAVTK QNVNAAMVFE FLYKMCDVM AAYFGKISEE NIKNNFVLIY ELLDEILDFG YPQNSETGAL KTFITQQGIK SQHQTKEEQS QITSQV(TPO) GQ IGWRREGIKY ...String: MIGGLFIYNH KGEVLISRVY RDDIGRNAVD AFRVNVIHAR QQVRSPVTNI ARTSFFHVKR SNIWLAAVTK QNVNAAMVFE FLYKMCDVM AAYFGKISEE NIKNNFVLIY ELLDEILDFG YPQNSETGAL KTFITQQGIK SQHQTKEEQS QITSQV(TPO) GQ IGWRREGIKY RRNELFLDVL ESVNLLMSPQ GQVLSAHVSG RVVMKSYLSG MPECKFGMND KIVIEKQGKG TADETSKS G KQSIAIDDCT FHQCVRLSKF DSERSISFIP PDGEFELMRY RTTKDIILPF RVIPLVREVG RTKLKVKVVI KSNFKPSLL AQKIEVRIPT PLNTSGVQVI CMKGKAKYKA SENAIVWKIK RMAGMKESQI SAEIELLPTN DKKKWARPPI SMNFEVPFAP SGLKVRYLK VFEPKLNYSD HDVIKWVRYI GRSGIYETRC UniProtKB: AP-2 complex subunit mu |
-Macromolecule #4: AP-2 complex subunit sigma
| Macromolecule | Name: AP-2 complex subunit sigma / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 17.038688 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIRFILIQNR AGKTRLAKWY MQFDDDEKQK LIEEVHAVVT VRDAKHTNFV EFRNFKIIYR RYAGLYFCIC VDVNDNNLAY LEAIHNFVE VLNEYFHNVC ELDLVFNFYK VYTVVDEMFL AGEIRETSQT KVLKQLLMLQ SLE UniProtKB: AP-2 complex subunit sigma |
-Macromolecule #5: Adaptin ear-binding coat-associated protein 2
| Macromolecule | Name: Adaptin ear-binding coat-associated protein 2 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 28.629941 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEESEYESVL CVKPEVHVYR IPPRATNRGY RASEWQLDQP SWSGRLRITA KGKVAYIKLE DRTSGELFAQ APVDQFPGTA VESVTDSSR YFVIRIEDGN GRRAFIGLGF GDRGDAFDFN VALQDHFKWV KQQCEFAKQA QNPDEGPKLD LGFKDGQTIK I NIANMRKK ...String: MEESEYESVL CVKPEVHVYR IPPRATNRGY RASEWQLDQP SWSGRLRITA KGKVAYIKLE DRTSGELFAQ APVDQFPGTA VESVTDSSR YFVIRIEDGN GRRAFIGLGF GDRGDAFDFN VALQDHFKWV KQQCEFAKQA QNPDEGPKLD LGFKDGQTIK I NIANMRKK EGAAGTPRAR PTSAGGLSLL PPPPGGKSST VIPPSGEQLS VGGSLVQPAV VSGSGGATEL WPQSKPAAAA TA DIWGDFT KSTGSPSSQS QPGTGWVQF UniProtKB: Adaptin ear-binding coat-associated protein 2 |
-Macromolecule #6: Unknown region of Adaptin ear-binding coat-associated protein 2 E...
| Macromolecule | Name: Unknown region of Adaptin ear-binding coat-associated protein 2 Ex-domain type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 613.749 Da |
| Recombinant expression | Organism:  |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: 4 UL OF SAMPLE/GRID WAS BLOTTED FOR 4 SECONDS AND PLUNGE FROZEN IN LIQUID-NITROGEN COOLED ETHANE. |
| Details | Contains an E302K mutation in the mu subunit of AP2. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 36000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | STARTING MODEL WAS GENERATED BY DOCKING PDB ENTRIES 2VGL AND 1TQZ INTO CRYO-EM DENSITY AND MANUALLY EDITING SEQUENCE AND STRUCTURAL CHANGES. MODEL REFINEMENT WAS PERFORMED USING ROSETTA AND PHENIX.REAL_SPACE_REFINE | ||||||
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||
| Output model |  PDB-6oxl: |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)