[English] 日本語
 Yorodumi
Yorodumi- EMDB-1495: Structural Analysis of the Saf Pilus by Electron Microscopy and I... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1495 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
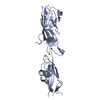
| Title | Structural Analysis of the Saf Pilus by Electron Microscopy and Image Processing. 3D reconstruction of the Type B Saf pilus, one of two conformations of the pilus refined. | |||||||||
 Map data Map data | 3D reconstruction of the Type B Saf pilus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Pilus / electron microscopy / image processing / single-particle analysis / atomic structure fitting | |||||||||
| Function / homology | Saf-pilin pilus formation protein / Saf-pilin pilus formation protein / Dr-adhesin superfamily / Adhesion domain superfamily / Prokaryotic membrane lipoprotein lipid attachment site profile. / Outer membrane protein Function and homology information Function and homology information | |||||||||
| Biological species |  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 17.0 Å | |||||||||
 Authors Authors | Salih O / Remaut H / Waksman G / Orlova EV | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2008 Journal: J Mol Biol / Year: 2008Title: Structural analysis of the Saf pilus by electron microscopy and image processing. Authors: Osman Salih / Han Remaut / Gabriel Waksman / Elena V Orlova /  Abstract: Bacterial pili are important virulence factors involved in host cell attachment and/or biofilm formation, key steps in establishing and maintaining successful infection. Here we studied Salmonella ...Bacterial pili are important virulence factors involved in host cell attachment and/or biofilm formation, key steps in establishing and maintaining successful infection. Here we studied Salmonella atypical fimbriae (or Saf pili), formed by the conserved chaperone/usher pathway. In contrast to the well-established quaternary structure of typical/FGS-chaperone assembled, rod-shaped, chaperone/usher pili, little is known about the supramolecular organisation in atypical/FGL-chaperone assembled fimbriae. In our study, we have used negative stain electron microscopy and single-particle image analysis to determine the three-dimensional structure of the Salmonella typhimurium Saf pilus. Our results show atypical/FGL-chaperone assembled fimbriae are composed of highly flexible linear multi-subunit fibres that are formed by globular subunits connected to each other by short links giving a "beads on a string"-like appearance. Quantitative fitting of the atomic structure of the SafA pilus subunit into the electron density maps, in combination with linker modelling and energy minimisation, has enabled analysis of subunit arrangement and intersubunit interactions in the Saf pilus. Short intersubunit linker regions provide the molecular basis for flexibility of the Saf pilus by acting as molecular hinges allowing a large range of movement between consecutive subunits in the fibre. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1495.map.gz emd_1495.map.gz | 849.4 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1495-v30.xml emd-1495-v30.xml emd-1495.xml emd-1495.xml | 9.5 KB 9.5 KB | Display Display |  EMDB header EMDB header |
| Images |  1495.gif 1495.gif | 28.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1495 http://ftp.pdbj.org/pub/emdb/structures/EMD-1495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1495 | HTTPS FTP |
-Related structure data
| Related structure data |  3crfMC  1494C  3creC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1495.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1495.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of the Type B Saf pilus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.591 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
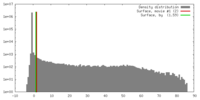
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Saf pilus
| Entire | Name: Saf pilus |
|---|---|
| Components |
|
-Supramolecule #1000: Saf pilus
| Supramolecule | Name: Saf pilus / type: sample / ID: 1000 / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 14 KDa |
-Macromolecule #1: Wild-type SafA
| Macromolecule | Name: Wild-type SafA / type: protein_or_peptide / ID: 1 / Name.synonym: SafA pilus subunit / Oligomeric state: Fibre / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria)Strain: LT2 / Location in cell: Cell surface |
| Molecular weight | Experimental: 14.4 KDa |
| Recombinant expression | Organism: Escherichia coli C600 / Recombinant plasmid: pTrc 99a |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 10 mM TRIS-HCl (pH 8) and 10 mM NaCl |
| Staining | Type: NEGATIVE / Details: 2% ammonium molybdate for 5 seconds |
| Grid | Details: Continuous-carbon film |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 1.591 µm / Number real images: 140 / Average electron dose: 25 e/Å2 / Od range: 1.5 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 100 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.4 mm / Nominal defocus max: 0.9 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 44000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: OTHER |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 17.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: Imagic / Number images used: 8500 |
|---|---|
| Final angle assignment | Details: Imagic: alpha, beta, gamma |
| Final two d classification | Number classes: 424 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)