[English] 日本語
 Yorodumi
Yorodumi- EMDB-11729: CryoEM structure of MIB-MIP in complex with a polyclonal goat Fab -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11729 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of MIB-MIP in complex with a polyclonal goat Fab | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Antibody binding protein / protease / protein complex. / MEMBRANE PROTEIN | |||||||||
| Function / homology | Uncharacterised protein family MG067 / Mycoplasma peptidase DUF31 / Mycoplasma peptidase (DUF31) / Mycoplasma virulence, signal domain / Putative immunoglobulin-blocking virulence protein / Mycoplasma virulence signal region (Myco_arth_vir_N) / IgG-blocking virulence domain / Putative immunoglobulin-blocking virulence protein / Lipoprotein Function and homology information Function and homology information | |||||||||
| Biological species |  Mycoplasma mycoides subsp. capri str. GM12 (bacteria) / Mycoplasma mycoides subsp. capri str. GM12 (bacteria) /  Mycoplasma mycoides subsp. capri (bacteria) Mycoplasma mycoides subsp. capri (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Nottelet P / Bataille L | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: The mycoplasma surface proteins MIB and MIP promote the dissociation of the antibody-antigen interaction. Authors: Pierre Nottelet / Laure Bataille / Geraldine Gourgues / Robin Anger / Carole Lartigue / Pascal Sirand-Pugnet / Esther Marza / Remi Fronzes / Yonathan Arfi /  Abstract: Mycoplasma immunoglobulin binding (MIB) and mycoplasma immunoglobulin protease (MIP) are surface proteins found in the majority of mycoplasma species, acting sequentially to capture antibodies and ...Mycoplasma immunoglobulin binding (MIB) and mycoplasma immunoglobulin protease (MIP) are surface proteins found in the majority of mycoplasma species, acting sequentially to capture antibodies and cleave off their V domains. Cryo-electron microscopy structures show how MIB and MIP bind to a Fab fragment in a "hug of death" mechanism. As a result, the orientation of the V and V domains is twisted out of alignment, disrupting the antigen binding site. We also show that MIB-MIP has the ability to promote the dissociation of the antibody-antigen complex. This system is functional in cells and protects mycoplasmas from antibody-mediated agglutination. These results highlight the key role of the MIB-MIP system in immunity evasion by mycoplasmas through an unprecedented mechanism, and open exciting perspectives to use these proteins as potential tools in the antibody field. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11729.map.gz emd_11729.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11729-v30.xml emd-11729-v30.xml emd-11729.xml emd-11729.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11729.png emd_11729.png | 47.8 KB | ||
| Filedesc metadata |  emd-11729.cif.gz emd-11729.cif.gz | 6.4 KB | ||
| Others |  emd_11729_additional_1.map.gz emd_11729_additional_1.map.gz emd_11729_half_map_1.map.gz emd_11729_half_map_1.map.gz emd_11729_half_map_2.map.gz emd_11729_half_map_2.map.gz | 49.4 MB 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11729 http://ftp.pdbj.org/pub/emdb/structures/EMD-11729 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11729 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11729 | HTTPS FTP |
-Related structure data
| Related structure data |  7adkMC  7adjC  7admC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11729.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11729.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
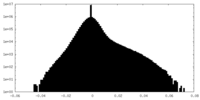
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_11729_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
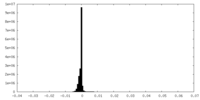
| Density Histograms |
-Half map: #1
| File | emd_11729_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
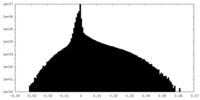
| Density Histograms |
-Half map: #2
| File | emd_11729_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
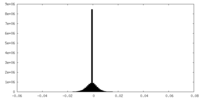
| Density Histograms |
- Sample components
Sample components
-Entire : Mycoplasma MIB-MIP proteins in complex with a goat Fab
| Entire | Name: Mycoplasma MIB-MIP proteins in complex with a goat Fab |
|---|---|
| Components |
|
-Supramolecule #1: Mycoplasma MIB-MIP proteins in complex with a goat Fab
| Supramolecule | Name: Mycoplasma MIB-MIP proteins in complex with a goat Fab type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycoplasma mycoides subsp. capri str. GM12 (bacteria) Mycoplasma mycoides subsp. capri str. GM12 (bacteria) |
-Macromolecule #1: Putative immunoglobulin-blocking virulence protein
| Macromolecule | Name: Putative immunoglobulin-blocking virulence protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycoplasma mycoides subsp. capri (bacteria) Mycoplasma mycoides subsp. capri (bacteria) |
| Molecular weight | Theoretical: 84.962719 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: VYFLKKKKNK ILTIALVASL AASVSFGSVL YYSFSDNHIS FDTSSNGITD AELAPINNAI NDAIVSNRDN KLKPSEEKII KETEKKIEE KIIIPPAKKE EKIEAAKPIP KPVVRKPETK ITSPKITRRK QTITIAGIEV EAEIEGPPGF VTHQRDKDRK I SNPTKPYQ ...String: VYFLKKKKNK ILTIALVASL AASVSFGSVL YYSFSDNHIS FDTSSNGITD AELAPINNAI NDAIVSNRDN KLKPSEEKII KETEKKIEE KIIIPPAKKE EKIEAAKPIP KPVVRKPETK ITSPKITRRK QTITIAGIEV EAEIEGPPGF VTHQRDKDRK I SNPTKPYQ NHTVNKILSV KVTDKLKEQV AKDALSGGNG YDEGVGLFNN SIFNVFKEEF NSGKELNDIL SSLESVARQN SG AFQNTLE RYKKMLDSNN VINFLKSEAQ KEYPKLKSKF QTKNQEYIWL IANLDQSKFT KIASTSEKYL EKGLTISPRS AFI NEAGEI DSNGWGPPDE YNTVTSRLRR DNSEYRVFDY DEYYSRSSDR IANGTYPGWV KEDVSEPYSK KYNFKASDGI RFSK LERIN PNPAKGKLNS GLVLDLDVSN DEAYRRSKEL IEKLQKDGEQ ITSYRIKNMG EKNSDQAFKD ILGALPKDIQ QLELF FSDK ATNTASLIAL ENKNIKELSL YTSGNSLKKA WSYNPLALRN TTWINTIDYN VSAEYSSHDK ITTRITFNTL AFDQED FSN GSYERINDGL RMVYYARNNE PFFQGGHGPG LEPDKKLGQN SYPTGLDFSR VTGIKSLKGL RFDDDLDTSN EPRKITE LT LYNNESYFEI SSDELNEANL QHLSTGEGNP EKPKIHFSNG NNTTSIRISG KTLLSDEGRR NLDKYFEYNE SLRNSGKQ I QIPNGSDELK KQLEGWGYKV STASDRSFT UniProtKB: Putative immunoglobulin-blocking virulence protein |
-Macromolecule #2: Lipoprotein
| Macromolecule | Name: Lipoprotein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycoplasma mycoides subsp. capri (bacteria) Mycoplasma mycoides subsp. capri (bacteria) |
| Molecular weight | Theoretical: 98.559016 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKRLNKLLMY ISSSTLLLPI TLLVACTPSK VVAKPINDDE FNKLIDSIKT ENDLLKYADI RFKNPNGADT KKEDIIPSQL KSENISITF KGKYQGQISA VVTNVDVDRT NPFAVQNEAT IFVQFKNLKT NTSKPISITI KGLNQKGNFD ASGNIVVDDF A YFGGTSGY ...String: MKRLNKLLMY ISSSTLLLPI TLLVACTPSK VVAKPINDDE FNKLIDSIKT ENDLLKYADI RFKNPNGADT KKEDIIPSQL KSENISITF KGKYQGQISA VVTNVDVDRT NPFAVQNEAT IFVQFKNLKT NTSKPISITI KGLNQKGNFD ASGNIVVDDF A YFGGTSGY DEYTKKDQKS RFDYDNERYM TRLKSQFGNS SNSINLKEYR GLETKQENIK KFDDQAAISN FDTYYNAALK GF TLPVYGS DGKVSGLKIY EGAEIGKGPS VVDSLGRNEK AKTVGLARTL PNEEYKTSAI QTFQTNFTIY KDYEKEIEEA EDN IKLFDS WNEQQIQSYI SAQLTQLRLN YEDEVSQIDR EISQTQPDKT TILSNLNQKK SKIESEYQKE LSTISKLNKD SLKE WQRKE IEKYNEKKKE KTFQISESGT MWIMDYLDEN AGKNPTKFYF GTNSHVAKGI KDGMVSFSLT RLNSEVKVGQ TFKLN GHDS NFTKFTFSPI NGNKLEDAVT AIFHATDFIN ENSSPLKLLD SEQKSKYNGA GIFADFAIVE VDFAKLLDKG KYSYSV WSA SNDITNQYET EQNKLISKIT NNYSESDKKV KFFSDSLLNE QTYAKFDRPL DFDPKKEDEL KKYNDLDSLY IVGYPTA YK DFYLDQYEDE KQLKNKKYDF SLWINSEYKF YNKLINKEGS TNSFKEYETG KGNFFSYQIG YRSFIDKPGL TDAFITVN K VGKKLYSLKD KNKNEVKKYF NYGLEILPRF YAPAGGASGS SVRTKDNKLL AVYHASNETA RTGLAVAFRS DGYDYKNLF GDYKLGQYDL IYGGGKDQQK EKSYREVMNK MYSGKKSALF QNGFTDDKIP SEFKFNNGTQ N UniProtKB: Lipoprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 4 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.45 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION / Number images used: 255911 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: RELION |
 Movie
Movie Controller
Controller














 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)