+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11092 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HDAC-PC | |||||||||
 Map data Map data | HDAC-PC | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Protein complex / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationHDA1 complex / : / HSF1 activation / HDACs deacetylate histones / nucleosome array spacer activity / histone deacetylase activity, hydrolytic mechanism / histone deacetylase / regulatory ncRNA-mediated gene silencing / SUMOylation of chromatin organization proteins / histone deacetylase complex ...HDA1 complex / : / HSF1 activation / HDACs deacetylate histones / nucleosome array spacer activity / histone deacetylase activity, hydrolytic mechanism / histone deacetylase / regulatory ncRNA-mediated gene silencing / SUMOylation of chromatin organization proteins / histone deacetylase complex / epigenetic regulation of gene expression / chromosome segregation / chromatin organization / chromatin binding / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / DNA binding / identical protein binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
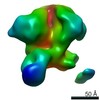
| Method | single particle reconstruction / cryo EM / Resolution: 3.11 Å | |||||||||
 Authors Authors | Lee J-H / Bollschweiler D | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Structural basis for the regulation of nucleosome recognition and HDAC activity by histone deacetylase assemblies. Authors: Jung-Hoon Lee / Daniel Bollschweiler / Tillman Schäfer / Robert Huber /  Abstract: The chromatin-modifying histone deacetylases (HDACs) remove acetyl groups from acetyl-lysine residues in histone amino-terminal tails, thereby mediating transcriptional repression. Structural makeup ...The chromatin-modifying histone deacetylases (HDACs) remove acetyl groups from acetyl-lysine residues in histone amino-terminal tails, thereby mediating transcriptional repression. Structural makeup and mechanisms by which multisubunit HDAC complexes recognize nucleosomes remain elusive. Our cryo-electron microscopy structures of the yeast class II HDAC ensembles show that the HDAC protomer comprises a triangle-shaped assembly of stoichiometry Hda1-Hda2-Hda3, in which the active sites of the Hda1 dimer are freely accessible. We also observe a tetramer of protomers, where the nucleosome binding modules are inaccessible. Structural analysis of the nucleosome-bound complexes indicates how positioning of Hda1 adjacent to histone H2B affords HDAC catalysis. Moreover, it reveals how an intricate network of multiple contacts between a dimer of protomers and the nucleosome creates a platform for expansion of the HDAC activities. Our study provides comprehensive insight into the structural plasticity of the HDAC complex and its functional mechanism of chromatin modification. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11092.map.gz emd_11092.map.gz | 62.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11092-v30.xml emd-11092-v30.xml emd-11092.xml emd-11092.xml | 15 KB 15 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11092.png emd_11092.png | 17.3 KB | ||
| Filedesc metadata |  emd-11092.cif.gz emd-11092.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11092 http://ftp.pdbj.org/pub/emdb/structures/EMD-11092 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11092 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11092 | HTTPS FTP |
-Related structure data
| Related structure data |  6z6fMC  6z6hC  6z6oC  6z6pC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11092.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11092.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HDAC-PC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.181 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : HDAC-PC
| Entire | Name: HDAC-PC |
|---|---|
| Components |
|
-Supramolecule #1: HDAC-PC
| Supramolecule | Name: HDAC-PC / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Histone deacetylase HDA1
| Macromolecule | Name: Histone deacetylase HDA1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: histone deacetylase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 74.851953 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RQVIVPVCMP KIHYSPLKTG LCYDVRMRYH AKIFTSYFEY IDPHPEDPRR IYRIYKILAE NGLINDPTLS GVDDLGDLML KIPVRAATS EEILEVHTKE HLEFIESTEK MSREELLKET EKGDSVYFNN DSYASARLPC GGAIEACKAV VEGRVKNSLA V VRPPGHHA ...String: RQVIVPVCMP KIHYSPLKTG LCYDVRMRYH AKIFTSYFEY IDPHPEDPRR IYRIYKILAE NGLINDPTLS GVDDLGDLML KIPVRAATS EEILEVHTKE HLEFIESTEK MSREELLKET EKGDSVYFNN DSYASARLPC GGAIEACKAV VEGRVKNSLA V VRPPGHHA EPQAAGGFCL FSNVAVAAKN ILKNYPESVR RIMILDWDIH HGNGTQKSFY QDDQVLYVSL HRFEMGKYYP GT IQGQYDQ TGEGKGEGFN CNITWPVGGV GDAEYMWAFE QVVMPMGREF KPDLVIISSG FDAADGDTIG QCHVTPSCYG HMT HMLKSL ARGNLCVVLE GGYNLDAIAR SALSVAKVLI GEPPDELPDP LSDPKPEVIE MIDKVIRLQS KYWNCFRRRH ANSG CNFNE PINDSIISKN FPLQKAIRQQ QQHYLSDEFN FVTLPLVSMD LPDNTVLCTP NISESNTIII VVHDTSDIWA KRNVI SGTI DLSSSVIIDN SLDFIKWGLD RKYGIIDVNI PLTLFEPDNY SGMITSQEVL IYLWDNYIKY FPSVAKIAFI GIGDSY SGI VHLLGHRDTR AVTKTVINFL GDKQLKPLVP LVDETLSEWY FKNSLIFSNN SHQCWKENES RKPRKKFGRV LRCDTDG LN NIIEERFEEA TDFILDSFE UniProtKB: Histone deacetylase HDA1 |
-Macromolecule #2: Histone deacetylase HDA1
| Macromolecule | Name: Histone deacetylase HDA1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: histone deacetylase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 76.017211 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ENSLSTTSKS KRQVIVPVCM PKIHYSPLKT GLCYDVRMRY HAKIFTSYFE YIDPHPEDPR RIYRIYKILA ENGLINDPTL SGVDDLGDL MLKIPVRAAT SEEILEVHTK EHLEFIESTE KMSREELLKE TEKGDSVYFN NDSYASARLP CGGAIEACKA V VEGRVKNS ...String: ENSLSTTSKS KRQVIVPVCM PKIHYSPLKT GLCYDVRMRY HAKIFTSYFE YIDPHPEDPR RIYRIYKILA ENGLINDPTL SGVDDLGDL MLKIPVRAAT SEEILEVHTK EHLEFIESTE KMSREELLKE TEKGDSVYFN NDSYASARLP CGGAIEACKA V VEGRVKNS LAVVRPPGHH AEPQAAGGFC LFSNVAVAAK NILKNYPESV RRIMILDWDI HHGNGTQKSF YQDDQVLYVS LH RFEMGKY YPGTIQGQYD QTGEGKGEGF NCNITWPVGG VGDAEYMWAF EQVVMPMGRE FKPDLVIISS GFDAADGDTI GQC HVTPSC YGHMTHMLKS LARGNLCVVL EGGYNLDAIA RSALSVAKVL IGEPPDELPD PLSDPKPEVI EMIDKVIRLQ SKYW NCFRR RHANSGCNFN EPINDSIISK NFPLQKAIRQ QQQHYLSDEF NFVTLPLVSM DLPDNTVLCT PNISESNTII IVVHD TSDI WAKRNVISGT IDLSSSVIID NSLDFIKWGL DRKYGIIDVN IPLTLFEPDN YSGMITSQEV LIYLWDNYIK YFPSVA KIA FIGIGDSYSG IVHLLGHRDT RAVTKTVINF LGDKQLKPLV PLVDETLSEW YFKNSLIFSN NSHQCWKENE SRKPRKK FG RVLRCDTDGL NNIIEERFEE ATDFILDSFE UniProtKB: Histone deacetylase HDA1 |
-Macromolecule #3: HDA1 complex subunit 3,HDA1 complex subunit 3
| Macromolecule | Name: HDA1 complex subunit 3,HDA1 complex subunit 3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 63.422098 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGDYWLPTTM SLYQKELTDQ IVSLHYSDIL RYFETSHYKE DVILESMKTM CLNGSLVATH PYLLIDHYMP KSLITRDVPA HLAENSGKF SVLRDLINLV QEYETETAIV CRPGRTMDLL EALLLGNKVH IKRYDGHSIK SKQKANDFSC TVHLFSSEGI N FTKYPIKS ...String: SGDYWLPTTM SLYQKELTDQ IVSLHYSDIL RYFETSHYKE DVILESMKTM CLNGSLVATH PYLLIDHYMP KSLITRDVPA HLAENSGKF SVLRDLINLV QEYETETAIV CRPGRTMDLL EALLLGNKVH IKRYDGHSIK SKQKANDFSC TVHLFSSEGI N FTKYPIKS KARFDMLICL DTTVDTSQKD IQYLLQYKRE RKGLERYAPI VRLVAINSID HCRLFFGKKF DKNSREYLEN VT AAMVILR DRLGTLPPDL RPIYSQKLHY LVEWLENPTV PWPLPDIYPL KQYTSMDVER SLLTEVHFKK NSSNVNYHLS SGI ITHKLI QSMGEVYMDI CVQKQELDDY SCLDDLQNDH LKFFSNEDEK IIKEYETVLR TNNENLNRSH ELEVENNLKF SQIE TLEKD IETLKGSLMA QGETLSKLKD AFVKTDNVQD EIEKEERVSV SRDTEKKYME QEIKRAVDAI RENEEETHKL NEKQN GLES ELKLKFEKSE ISTKELNEKI GFLKKELKLE NDLNEELVGQ LSKTMDNLEN LTIPRVRTQ UniProtKB: HDA1 complex subunit 3, HDA1 complex subunit 3 |
-Macromolecule #4: HDA1 complex subunit 2
| Macromolecule | Name: HDA1 complex subunit 2 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 71.915297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KVYYLPVTLT QFQKDLSEIL ISLHAKSFKA SIIGEPQADA VNKPSGLPAG PETHPYPTLS QRQLTYIFDS NIRAIANHPS LLVDHYMPR QLLRMEPTES SIAGSHKFQV LNQLINSICF RDREGSPNEV IKCAIIAHSI KELDLLEGLI LGKKFRTKRL S GTSLYNEK ...String: KVYYLPVTLT QFQKDLSEIL ISLHAKSFKA SIIGEPQADA VNKPSGLPAG PETHPYPTLS QRQLTYIFDS NIRAIANHPS LLVDHYMPR QLLRMEPTES SIAGSHKFQV LNQLINSICF RDREGSPNEV IKCAIIAHSI KELDLLEGLI LGKKFRTKRL S GTSLYNEK HKFPNLPTVD STINKDGTPN SVSSTSSNSN STSYTGYSKD DYDYSVKRNL KKRKINTDDW LFLATTKHLK HD QYLLANY DIDMIISFDP MLEVELPALQ VLRNNANKDI PIIKLLVQNS PDHYLLDSEI KNSSVKSSHL SNNGHVDDSQ EYE EIKSSL LYFLQARNAP VNNCEIDYIK LVKCCLEGKD CNNILPVLDL ITLDEASKDS SDSGFWQPQL TKLQYSSTEL PLWD GPLDI KTYQTELMHR AVIRLRDIQD EYAKGTVPLY EKRLNETQRQ NQLDEIKNSV GLTFKKKQEV EKSINDSEKR LKHAM TEST KLQNKINHLL KNRQELENFN KLPSNTISSE NHLEEGSALA DKLKEYIDKN ATLFNKLKEL QQANAEKSKL NDELRS KYQ IESSKAAESA QTLKILQESM KSLENEVNGP LTKFSTESLK KELERLQNDF QSLKARNKFL KNYITL UniProtKB: HDA1 complex subunit 2 |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 62.1 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.11 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2) / Number images used: 466972 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
-Atomic model buiding 1
| Details | Real space refinement |
|---|---|
| Output model |  PDB-6z6f: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















