[English] 日本語
 Yorodumi
Yorodumi- EMDB-10479: Tail-baseplate interface of native GTA particle computed with C6 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10479 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tail-baseplate interface of native GTA particle computed with C6 symmetry | ||||||||||||||||||||||||
 Map data Map data | tail-baseplate interface of native GTA particle computed with C6 symmetry | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | "distal tail protein" / "tail tube" / "oligosaccharide-binding fold" / "tail tube protein" / VIRUS | ||||||||||||||||||||||||
| Function / homology | Protein of unknown function DUF2460 / Conserved hypothetical protein 2217 (DUF2460) / Gene transfer agent, major tail protein / Phage major tail protein TP901-1 / Phage tail tube protein / Phage major tail protein, TP901-1 family / DUF2460 domain-containing protein Function and homology information Function and homology information | ||||||||||||||||||||||||
| Biological species |  Rhodobacter capsulatus DE442 (bacteria) Rhodobacter capsulatus DE442 (bacteria) | ||||||||||||||||||||||||
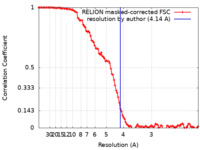
| Method | single particle reconstruction / cryo EM / Resolution: 4.14 Å | ||||||||||||||||||||||||
 Authors Authors | Bardy P / Fuzik T | ||||||||||||||||||||||||
| Funding support |  Czech Republic, 7 items Czech Republic, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structure and mechanism of DNA delivery of a gene transfer agent. Authors: Pavol Bárdy / Tibor Füzik / Dominik Hrebík / Roman Pantůček / J Thomas Beatty / Pavel Plevka /   Abstract: Alphaproteobacteria, which are the most abundant microorganisms of temperate oceans, produce phage-like particles called gene transfer agents (GTAs) that mediate lateral gene exchange. However, the ...Alphaproteobacteria, which are the most abundant microorganisms of temperate oceans, produce phage-like particles called gene transfer agents (GTAs) that mediate lateral gene exchange. However, the mechanism by which GTAs deliver DNA into cells is unknown. Here we present the structure of the GTA of Rhodobacter capsulatus (RcGTA) and describe the conformational changes required for its DNA ejection. The structure of RcGTA resembles that of a tailed phage, but it has an oblate head shortened in the direction of the tail axis, which limits its packaging capacity to less than 4,500 base pairs of linear double-stranded DNA. The tail channel of RcGTA contains a trimer of proteins that possess features of both tape measure proteins of long-tailed phages from the family Siphoviridae and tail needle proteins of short-tailed phages from the family Podoviridae. The opening of a constriction within the RcGTA baseplate enables the ejection of DNA into bacterial periplasm. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10479.map.gz emd_10479.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10479-v30.xml emd-10479-v30.xml emd-10479.xml emd-10479.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10479_fsc.xml emd_10479_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_10479.png emd_10479.png | 131.5 KB | ||
| Filedesc metadata |  emd-10479.cif.gz emd-10479.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10479 http://ftp.pdbj.org/pub/emdb/structures/EMD-10479 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10479 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10479 | HTTPS FTP |
-Related structure data
| Related structure data |  6tebMC  6tb9C  6tbaC  6te8C  6te9C  6teaC  6tehC  6to8C  6toaC  6tsuC  6tsvC  6tswC  6tuiC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10479.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10479.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | tail-baseplate interface of native GTA particle computed with C6 symmetry | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.063 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Rhodobacter capsulatus DE442 gene transfer agent baseplate-tail i...
| Entire | Name: Rhodobacter capsulatus DE442 gene transfer agent baseplate-tail interface |
|---|---|
| Components |
|
-Supramolecule #1: Rhodobacter capsulatus DE442 gene transfer agent baseplate-tail i...
| Supramolecule | Name: Rhodobacter capsulatus DE442 gene transfer agent baseplate-tail interface type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: region connecting the tail tube with host-recognition device (baseplate), last disk of tail tube and distal tail protein is shown |
|---|---|
| Source (natural) | Organism:  Rhodobacter capsulatus DE442 (bacteria) Rhodobacter capsulatus DE442 (bacteria) |
-Macromolecule #1: Tail tube protein Rcc01691
| Macromolecule | Name: Tail tube protein Rcc01691 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhodobacter capsulatus DE442 (bacteria) Rhodobacter capsulatus DE442 (bacteria) |
| Molecular weight | Theoretical: 14.420007 KDa |
| Sequence | String: MAAQNGKDLL IKLDLTGSGQ FETIAGLRAT RISFNAETVD VTSLESQGGW RELLGGAGVR SASISGAGVF KDADTDERAR QIFFDGEVP EFQVIIPDFG IVQGPFMITS IDYAGSHNGE ASYELAMASA GALSFTAI UniProtKB: Phage major tail protein, TP901-1 family |
-Macromolecule #2: Distal tail protein Rcc01695
| Macromolecule | Name: Distal tail protein Rcc01695 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhodobacter capsulatus DE442 (bacteria) Rhodobacter capsulatus DE442 (bacteria) |
| Molecular weight | Theoretical: 22.985713 KDa |
| Sequence | String: MAFHEVRFPA NLSFGSVGGP ERRTEIVTLS SGHEERNSPW AHSRRHYDAG VGLRSLDDVE RLIAFFEARG GQLHGFRWKD WADFKSCPA SRAVAHEDQL IGMGDGVTTA FQLVKTYVSG GQSYLRPIVK PVEGTVKLGI AGDHQAEAVN FAVDHATGIV S FNEPPPQG ...String: MAFHEVRFPA NLSFGSVGGP ERRTEIVTLS SGHEERNSPW AHSRRHYDAG VGLRSLDDVE RLIAFFEARG GQLHGFRWKD WADFKSCPA SRAVAHEDQL IGMGDGVTTA FQLVKTYVSG GQSYLRPIVK PVEGTVKLGI AGDHQAEAVN FAVDHATGIV S FNEPPPQG ARVTAGFEFD VPVRFDTDRI AVSVQSFQAG DLPQVPVVEV RI UniProtKB: DUF2460 domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 20 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 Component:
Details: G-buffer, doi: 10.1016/0003-9861(77)90508-2 | ||||||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 11 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 3114 / Average exposure time: 1.0 sec. / Average electron dose: 42.75 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -3.0 µm / Nominal defocus min: -1.0 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6teb: |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)