[English] 日本語
 Yorodumi
Yorodumi- PDB-1abo: CRYSTAL STRUCTURE OF THE COMPLEX OF THE ABL TYROSINE KINASE SH3 D... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1abo | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THE COMPLEX OF THE ABL TYROSINE KINASE SH3 DOMAIN WITH 3BP-1 SYNTHETIC PEPTIDE | ||||||
 Components Components |
| ||||||
 Keywords Keywords | COMPLEX (KINASE/PEPTIDE) / SH3 DOMAIN / TRANSFERASE (PHOSPHOTRANSFERASE) / PROTO-ONCOGENE / COMPLEX (KINASE-PEPTIDE) COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of actin filament depolymerization / Role of ABL in ROBO-SLIT signaling / HDR through Single Strand Annealing (SSA) / negative regulation of small GTPase mediated signal transduction / exocyst / RHO GTPases Activate WASPs and WAVEs / semaphorin receptor binding / Cyclin D associated events in G1 / RAC1 GTPase cycle / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks ...regulation of actin filament depolymerization / Role of ABL in ROBO-SLIT signaling / HDR through Single Strand Annealing (SSA) / negative regulation of small GTPase mediated signal transduction / exocyst / RHO GTPases Activate WASPs and WAVEs / semaphorin receptor binding / Cyclin D associated events in G1 / RAC1 GTPase cycle / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / protein localization to cytoplasmic microtubule plus-end / DN4 thymocyte differentiation / regulation of cellular senescence / response to epinephrine / RUNX1 regulates transcription of genes involved in differentiation of HSCs / podocyte apoptotic process / transitional one stage B cell differentiation / regulation of modification of synaptic structure / Regulation of actin dynamics for phagocytic cup formation / regulation of Rac protein signal transduction / delta-catenin binding / DNA conformation change / ruffle assembly / microspike assembly / neuroepithelial cell differentiation / B cell proliferation involved in immune response / positive regulation of Wnt signaling pathway, planar cell polarity pathway / positive regulation of extracellular matrix organization / cerebellum morphogenesis / regulation of extracellular matrix organization / positive regulation of blood vessel branching / circulatory system development / regulation of blood vessel endothelial cell migration / B-1 B cell homeostasis / neuropilin signaling pathway / neuropilin binding / Myogenesis / bubble DNA binding / cell junction assembly / filopodium assembly / establishment of epithelial cell apical/basal polarity / activated T cell proliferation / regulation of Cdc42 protein signal transduction / proline-rich region binding / mitogen-activated protein kinase binding / positive regulation of dendrite development / syntaxin binding / myoblast proliferation / alpha-beta T cell differentiation / regulation of T cell differentiation / cardiac muscle cell proliferation / regulation of axon extension / positive regulation of cell migration involved in sprouting angiogenesis / phagocytosis, engulfment / negative regulation of cell-cell adhesion / B cell proliferation / cell leading edge / positive regulation of osteoblast proliferation / regulation of microtubule polymerization / platelet-derived growth factor receptor-beta signaling pathway / negative regulation of cellular senescence / positive regulation of focal adhesion assembly / associative learning / Bergmann glial cell differentiation / platelet-derived growth factor receptor signaling pathway / semaphorin-plexin signaling pathway / neuromuscular process controlling balance / phagocytic cup / negative regulation of mitotic cell cycle / negative regulation of long-term synaptic potentiation / negative regulation of BMP signaling pathway / negative regulation of double-strand break repair via homologous recombination / endothelial cell migration / bicellular tight junction / signal transduction in response to DNA damage / positive regulation of T cell migration / BMP signaling pathway / negative regulation of endothelial cell apoptotic process / canonical NF-kappaB signal transduction / phagocytosis / positive regulation of substrate adhesion-dependent cell spreading / four-way junction DNA binding / spleen development / cellular response to transforming growth factor beta stimulus / positive regulation of vasoconstriction / positive regulation of stress fiber assembly / ruffle / positive regulation of establishment of T cell polarity / phosphotyrosine residue binding / response to endoplasmic reticulum stress / ephrin receptor binding / positive regulation of interleukin-2 production / actin filament polymerization / ERK1 and ERK2 cascade / post-embryonic development / GTPase activator activity / SH2 domain binding / positive regulation of mitotic cell cycle / substrate adhesion-dependent cell spreading / positive regulation of GTPase activity Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2 Å X-RAY DIFFRACTION / Resolution: 2 Å | ||||||
 Authors Authors | Musacchio, A. / Wilmanns, M. / Saraste, M. | ||||||
 Citation Citation |  Journal: Nat.Struct.Biol. / Year: 1994 Journal: Nat.Struct.Biol. / Year: 1994Title: High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Authors: Musacchio, A. / Saraste, M. / Wilmanns, M. #1:  Journal: Embo J. / Year: 1993 Journal: Embo J. / Year: 1993Title: Crystal Structure of the SH3 Domain in Human Fyn. Comparison of the Three-Dimensional Structures of the SH3 Domain in Tyrosine Kinases and Spectrin Authors: Noble, M.E.M. / Musacchio, A. / Courtneidge, S. / Saraste, M. / Wierenga, R. #2:  Journal: Science / Year: 1993 Journal: Science / Year: 1993Title: Identification of a Ten-Amino Acid SH3 Binding Site Authors: Ren, R. / Mayer, B. / Clark, K.L. / Baltimore, D. #3:  Journal: Nature / Year: 1992 Journal: Nature / Year: 1992Title: Crystal Structure of a Src-Homology 3 (SH3) Domain Authors: Musacchio, A. / Noble, M.E.M. / Pauptit, R. / Wierenga, R. / Saraste, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1abo.cif.gz 1abo.cif.gz | 40.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1abo.ent.gz pdb1abo.ent.gz | 28.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1abo.json.gz 1abo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1abo_validation.pdf.gz 1abo_validation.pdf.gz | 386.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1abo_full_validation.pdf.gz 1abo_full_validation.pdf.gz | 386.6 KB | Display | |
| Data in XML |  1abo_validation.xml.gz 1abo_validation.xml.gz | 4.1 KB | Display | |
| Data in CIF |  1abo_validation.cif.gz 1abo_validation.cif.gz | 6.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ab/1abo https://data.pdbj.org/pub/pdb/validation_reports/ab/1abo ftp://data.pdbj.org/pub/pdb/validation_reports/ab/1abo ftp://data.pdbj.org/pub/pdb/validation_reports/ab/1abo | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 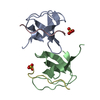
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 6880.580 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Protein/peptide | Mass: 1017.239 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source References: UniProt: P55194 #3: Chemical | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.01 Å3/Da / Density % sol: 38.9 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 8.2 / Method: vapor diffusion, hanging drop / Details: used as seeds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Wavelength: 1.5418 Å |
|---|---|
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Dec 20, 1993 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | % possible obs: 89 % / Rmerge(I) obs: 0.029 |
| Reflection | *PLUS Highest resolution: 2 Å / Rmerge(I) obs: 0.029 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2→8 Å / σ(F): 1 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj











