[English] 日本語
 Yorodumi
Yorodumi- PDB-6tcz: Leishmania tarentolae proteasome 20S subunit complexed with LXE408 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6tcz | ||||||
|---|---|---|---|---|---|---|---|
| Title | Leishmania tarentolae proteasome 20S subunit complexed with LXE408 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE / Proteasome complex / inhibitor / peptidase | ||||||
| Function / homology |  Function and homology information Function and homology informationphosphoenolpyruvate carboxykinase activity / proteasome core complex / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / threonine-type endopeptidase activity / proteasome core complex, alpha-subunit complex / proteasomal protein catabolic process / : / gluconeogenesis / ubiquitin-dependent protein catabolic process ...phosphoenolpyruvate carboxykinase activity / proteasome core complex / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / threonine-type endopeptidase activity / proteasome core complex, alpha-subunit complex / proteasomal protein catabolic process / : / gluconeogenesis / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / protein kinase activity / hydrolase activity / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Leishmania donovani (eukaryote) Leishmania donovani (eukaryote) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||
 Authors Authors | Srinivas, H. | ||||||
 Citation Citation |  Journal: J Med Chem / Year: 2020 Journal: J Med Chem / Year: 2020Title: Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. Authors: Advait Nagle / Agnes Biggart / Celine Be / Honnappa Srinivas / Andreas Hein / Diana Caridha / Richard J Sciotti / Brandon Pybus / Mara Kreishman-Deitrick / Badry Bursulaya / Yin H Lai / Mu- ...Authors: Advait Nagle / Agnes Biggart / Celine Be / Honnappa Srinivas / Andreas Hein / Diana Caridha / Richard J Sciotti / Brandon Pybus / Mara Kreishman-Deitrick / Badry Bursulaya / Yin H Lai / Mu-Yun Gao / Fang Liang / Casey J N Mathison / Xiaodong Liu / Vince Yeh / Jeffrey Smith / Isabelle Lerario / Yongping Xie / Donatella Chianelli / Michael Gibney / Ashley Berman / Yen-Liang Chen / Jan Jiricek / Lauren C Davis / Xianzhong Liu / Jaime Ballard / Shilpi Khare / Fabian Kurt Eggimann / Alexandre Luneau / Todd Groessl / Michael Shapiro / Wendy Richmond / Kevin Johnson / Patrick J Rudewicz / Srinivasa P S Rao / Christopher Thompson / Tove Tuntland / Glen Spraggon / Richard J Glynne / Frantisek Supek / Christian Wiesmann / Valentina Molteni /   Abstract: Visceral leishmaniasis is responsible for up to 30,000 deaths every year. Current treatments have shortcomings that include toxicity and variable efficacy across endemic regions. Previously, we ...Visceral leishmaniasis is responsible for up to 30,000 deaths every year. Current treatments have shortcomings that include toxicity and variable efficacy across endemic regions. Previously, we reported the discovery of GNF6702, a selective inhibitor of the kinetoplastid proteasome, which cleared parasites in murine models of leishmaniasis, Chagas disease, and human African trypanosomiasis. Here, we describe the discovery and characterization of LXE408, a structurally related kinetoplastid-selective proteasome inhibitor currently in Phase 1 human clinical trials. Furthermore, we present high-resolution cryo-EM structures of the proteasome in complex with LXE408, which provides a compelling explanation for the noncompetitive mode of binding of this novel class of inhibitors of the kinetoplastid proteasome. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6tcz.cif.gz 6tcz.cif.gz | 1.1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6tcz.ent.gz pdb6tcz.ent.gz | 888.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6tcz.json.gz 6tcz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tc/6tcz https://data.pdbj.org/pub/pdb/validation_reports/tc/6tcz ftp://data.pdbj.org/pub/pdb/validation_reports/tc/6tcz ftp://data.pdbj.org/pub/pdb/validation_reports/tc/6tcz | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10462MC  6td5C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Proteasome subunit ... , 12 types, 24 molecules AaBbCcEeFfHhIiJjKkLlMmNn
| #1: Protein | Mass: 27178.107 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: Pa, CGC20_14335, CGC21_7870, LdCL_350054100 / Production host: Leishmania donovani (eukaryote) / Gene: Pa, CGC20_14335, CGC21_7870, LdCL_350054100 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: Q9UAB4*PLUS, proteasome endopeptidase complex #2: Protein | Mass: 25179.559 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_21935, CGC21_34645, LdCL_210026400 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_21935, CGC21_34645, LdCL_210026400 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3Q8IB07*PLUS, proteasome endopeptidase complex #3: Protein | Mass: 32321.438 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC21_4565, LdCL_140008100 / Production host: Leishmania donovani (eukaryote) / Gene: CGC21_4565, LdCL_140008100 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A504Y5E1*PLUS, proteasome endopeptidase complex #5: Protein | Mass: 38312.316 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_21875, CGC21_34705, LdCL_210027700 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_21875, CGC21_34705, LdCL_210027700 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3Q8IC41*PLUS, proteasome endopeptidase complex #6: Protein | Mass: 47978.633 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_29090, CGC21_13150 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_29090, CGC21_13150 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A504XQ80*PLUS, proteasome endopeptidase complex #8: Protein | Mass: 30280.010 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_24015, CGC21_10040 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_24015, CGC21_10040 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A504X6A3*PLUS, proteasome endopeptidase complex #9: Protein | Mass: 27603.570 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_14810, CGC21_7390, LdCL_350043900 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_14810, CGC21_7390, LdCL_350043900 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3Q8IVH0*PLUS, proteasome endopeptidase complex #10: Protein | Mass: 22470.887 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_14015, CGC21_21605, LdCL_280006000 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_14015, CGC21_21605, LdCL_280006000 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3S7X127*PLUS, proteasome endopeptidase complex #11: Protein | Mass: 23065.291 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC21_12505 / Production host: Leishmania donovani (eukaryote) / Gene: CGC21_12505 / Production host:  Leishmania tarentolae (eukaryote) / References: UniProt: A0A504XH29*PLUS Leishmania tarentolae (eukaryote) / References: UniProt: A0A504XH29*PLUS#12: Protein | Mass: 33704.867 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_29060, CGC21_13180, LdCL_360022800 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_29060, CGC21_13180, LdCL_360022800 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3Q8IIY4*PLUS, proteasome endopeptidase complex #13: Protein | Mass: 37676.910 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_20280, CGC21_24485, LdCL_060006300 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_20280, CGC21_24485, LdCL_060006300 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3S7WPD8*PLUS, proteasome endopeptidase complex #14: Protein | Mass: 24737.232 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) Leishmania donovani (eukaryote)Gene: CGC21_27345, CGC21_27360, LdCL_340051000, LdCL_340051300 Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3Q8IIL6*PLUS, proteasome endopeptidase complex |
|---|
-Proteasome endopeptidase ... , 2 types, 4 molecules DdGg
| #4: Protein | Mass: 27821.605 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC21_1080 / Production host: Leishmania donovani (eukaryote) / Gene: CGC21_1080 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A504XWY9*PLUS, proteasome endopeptidase complex #7: Protein | Mass: 25591.826 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Leishmania donovani (eukaryote) / Gene: CGC20_1425, CGC20_33255, CGC21_31345, LdCL_270006800 / Production host: Leishmania donovani (eukaryote) / Gene: CGC20_1425, CGC20_33255, CGC21_31345, LdCL_270006800 / Production host:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote)References: UniProt: A0A3S5H7H2*PLUS, proteasome endopeptidase complex |
|---|
-Non-polymers , 1 types, 2 molecules 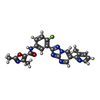
| #15: Chemical |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Leishmania tarentolae proteasome 20S subunit complex / Type: COMPLEX / Entity ID: #1-#14 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Leishmania donovani (eukaryote) Leishmania donovani (eukaryote) |
| Source (recombinant) | Organism:  Leishmania tarentolae (eukaryote) Leishmania tarentolae (eukaryote) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 3 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 1 e/Å2 / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| CTF correction | Type: NONE |
|---|---|
| Symmetry | Point symmetry: C2 (2 fold cyclic) |
| 3D reconstruction | Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 38000 / Symmetry type: POINT |
 Movie
Movie Controller
Controller
























 PDBj
PDBj




