+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ot0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of human Smoothened-Gi complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SIGNALING PROTEIN / GPCR / Complex / Hedgehog signaling | ||||||
| Function / homology |  Function and homology information Function and homology informationventral midline determination / mesenchymal to epithelial transition involved in metanephric renal vesicle formation / response to inositol / regulation of heart morphogenesis / contact inhibition / negative regulation of hair follicle development / 9+0 non-motile cilium / pancreas morphogenesis / regulation of somatic stem cell population maintenance / epithelial-mesenchymal cell signaling ...ventral midline determination / mesenchymal to epithelial transition involved in metanephric renal vesicle formation / response to inositol / regulation of heart morphogenesis / contact inhibition / negative regulation of hair follicle development / 9+0 non-motile cilium / pancreas morphogenesis / regulation of somatic stem cell population maintenance / epithelial-mesenchymal cell signaling / myoblast migration / atrial septum morphogenesis / spinal cord dorsal/ventral patterning / determination of left/right asymmetry in lateral mesoderm / midgut development / left/right axis specification / negative regulation of DNA binding / patched binding / somite development / forebrain morphogenesis / type B pancreatic cell development / ciliary tip / Activation of SMO / BBSome-mediated cargo-targeting to cilium / positive regulation of organ growth / smooth muscle tissue development / dorsal/ventral neural tube patterning / cerebellar cortex morphogenesis / positive regulation of branching involved in ureteric bud morphogenesis / mammary gland epithelial cell differentiation / cellular response to cholesterol / commissural neuron axon guidance / dentate gyrus development / pattern specification process / oxysterol binding / dopaminergic neuron differentiation / thalamus development / positive regulation of multicellular organism growth / positive regulation of smoothened signaling pathway / Class B/2 (Secretin family receptors) / cell fate specification / cAMP-dependent protein kinase inhibitor activity / central nervous system neuron differentiation / neural crest cell migration / anterior/posterior pattern specification / positive regulation of mesenchymal cell proliferation / hair follicle morphogenesis / ciliary membrane / smoothened signaling pathway / positive regulation of neuroblast proliferation / negative regulation of epithelial cell differentiation / heart looping / endoplasmic reticulum-Golgi intermediate compartment / protein kinase A catalytic subunit binding / odontogenesis of dentin-containing tooth / negative regulation of protein phosphorylation / neuroblast proliferation / vasculogenesis / Hedgehog 'off' state / adenylate cyclase inhibitor activity / skeletal muscle fiber development / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / astrocyte activation / centriole / homeostasis of number of cells within a tissue / protein sequestering activity / cellular response to forskolin / regulation of mitotic spindle organization / central nervous system development / epithelial cell proliferation / positive regulation of epithelial cell proliferation / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / Hedgehog 'on' state / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / G protein-coupled receptor activity / cerebral cortex development / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / positive regulation of protein import into nucleus / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / multicellular organism growth / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / protein import into nucleus / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / Resolution: 3.84 Å | ||||||
 Authors Authors | Qi, X. / Li, X. | ||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric G. Authors: Xiaofeng Qi / Heng Liu / Bonne Thompson / Jeffrey McDonald / Cheng Zhang / Xiaochun Li /  Abstract: The oncoprotein Smoothened (SMO), a G-protein-coupled receptor (GPCR) of the Frizzled-class (class-F), transduces the Hedgehog signal from the tumour suppressor Patched-1 (PTCH1) to the glioma- ...The oncoprotein Smoothened (SMO), a G-protein-coupled receptor (GPCR) of the Frizzled-class (class-F), transduces the Hedgehog signal from the tumour suppressor Patched-1 (PTCH1) to the glioma-associated-oncogene (GLI) transcription factors, which activates the Hedgehog signalling pathway. It has remained unknown how PTCH1 modulates SMO, how SMO is stimulated to form a complex with heterotrimeric G proteins and whether G-protein coupling contributes to the activation of GLI proteins. Here we show that 24,25-epoxycholesterol, which we identify as an endogenous ligand of PTCH1, can stimulate Hedgehog signalling in cells and can trigger G-protein signalling via human SMO in vitro. We present a cryo-electron microscopy structure of human SMO bound to 24(S),25-epoxycholesterol and coupled to a heterotrimeric G protein. The structure reveals a ligand-binding site for 24(S),25-epoxycholesterol in the 7-transmembrane region, as well as a G-coupled activation mechanism of human SMO. Notably, the G protein presents a different arrangement from that of class-A GPCR-G complexes. Our work provides molecular insights into Hedgehog signal transduction and the activation of a class-F GPCR. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ot0.cif.gz 6ot0.cif.gz | 286 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ot0.ent.gz pdb6ot0.ent.gz | 220.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ot0.json.gz 6ot0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ot/6ot0 https://data.pdbj.org/pub/pdb/validation_reports/ot/6ot0 ftp://data.pdbj.org/pub/pdb/validation_reports/ot/6ot0 ftp://data.pdbj.org/pub/pdb/validation_reports/ot/6ot0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  20190MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules ABG
| #2: Protein | Mass: 40414.047 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAI1 / Production host: Homo sapiens (human) / Gene: GNAI1 / Production host:  |
|---|---|
| #3: Protein | Mass: 38744.371 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Production host: Homo sapiens (human) / Gene: GNB1 / Production host:  |
| #4: Protein | Mass: 7861.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Production host: Homo sapiens (human) / Gene: GNG2 / Production host:  |
-Antibody , 2 types, 2 molecules LH
| #5: Antibody | Mass: 23258.783 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #6: Antibody | Mass: 25788.822 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Protein / Non-polymers , 2 types, 2 molecules R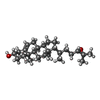

| #1: Protein | Mass: 62109.387 Da / Num. of mol.: 1 / Fragment: residues 1-555 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SMO, SMOH / Production host: Homo sapiens (human) / Gene: SMO, SMOH / Production host:  Homo sapiens (human) / References: UniProt: Q99835 Homo sapiens (human) / References: UniProt: Q99835 |
|---|---|
| #7: Chemical | ChemComp-CO1 / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.250 MDa / Experimental value: YES | ||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: NO |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: DARK FIELD |
| Image recording | Electron dose: 1.4 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.8.0158 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.84 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 141100 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 3.84→3.84 Å / Cor.coef. Fo:Fc: 0.876 / SU B: 148.702 / SU ML: 1.573 / ESU R: 0.975 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 200.285 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 10572 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


























