+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20190 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human Smoothened-Gi complex | |||||||||
 Map data Map data | Smoothened-Gi complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / Complex / Hedgehog signaling / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationventral midline determination / mesenchymal to epithelial transition involved in metanephric renal vesicle formation / response to inositol / regulation of heart morphogenesis / contact inhibition / negative regulation of hair follicle development / 9+0 non-motile cilium / regulation of somatic stem cell population maintenance / pancreas morphogenesis / epithelial-mesenchymal cell signaling ...ventral midline determination / mesenchymal to epithelial transition involved in metanephric renal vesicle formation / response to inositol / regulation of heart morphogenesis / contact inhibition / negative regulation of hair follicle development / 9+0 non-motile cilium / regulation of somatic stem cell population maintenance / pancreas morphogenesis / epithelial-mesenchymal cell signaling / myoblast migration / atrial septum morphogenesis / spinal cord dorsal/ventral patterning / determination of left/right asymmetry in lateral mesoderm / midgut development / left/right axis specification / negative regulation of DNA binding / patched binding / somite development / forebrain morphogenesis / type B pancreatic cell development / ciliary tip / Activation of SMO / BBSome-mediated cargo-targeting to cilium / positive regulation of organ growth / dorsal/ventral neural tube patterning / smooth muscle tissue development / cerebellar cortex morphogenesis / mammary gland epithelial cell differentiation / positive regulation of branching involved in ureteric bud morphogenesis / cellular response to cholesterol / dentate gyrus development / commissural neuron axon guidance / pattern specification process / oxysterol binding / dopaminergic neuron differentiation / thalamus development / positive regulation of multicellular organism growth / positive regulation of smoothened signaling pathway / Class B/2 (Secretin family receptors) / cell fate specification / cAMP-dependent protein kinase inhibitor activity / central nervous system neuron differentiation / anterior/posterior pattern specification / neural crest cell migration / positive regulation of mesenchymal cell proliferation / hair follicle morphogenesis / ciliary membrane / smoothened signaling pathway / positive regulation of neuroblast proliferation / negative regulation of epithelial cell differentiation / endoplasmic reticulum-Golgi intermediate compartment / heart looping / protein kinase A catalytic subunit binding / odontogenesis of dentin-containing tooth / negative regulation of protein phosphorylation / neuroblast proliferation / vasculogenesis / Hedgehog 'off' state / adenylate cyclase inhibitor activity / skeletal muscle fiber development / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / centriole / astrocyte activation / homeostasis of number of cells within a tissue / protein sequestering activity / cellular response to forskolin / regulation of mitotic spindle organization / epithelial cell proliferation / central nervous system development / positive regulation of epithelial cell proliferation / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / Hedgehog 'on' state / negative regulation of insulin secretion / G protein-coupled receptor binding / cerebral cortex development / response to peptide hormone / G protein-coupled receptor activity / positive regulation of protein import into nucleus / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / multicellular organism growth / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / protein import into nucleus / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / Resolution: 3.84 Å | |||||||||
 Authors Authors | Qi X / Li X | |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric G. Authors: Xiaofeng Qi / Heng Liu / Bonne Thompson / Jeffrey McDonald / Cheng Zhang / Xiaochun Li /  Abstract: The oncoprotein Smoothened (SMO), a G-protein-coupled receptor (GPCR) of the Frizzled-class (class-F), transduces the Hedgehog signal from the tumour suppressor Patched-1 (PTCH1) to the glioma- ...The oncoprotein Smoothened (SMO), a G-protein-coupled receptor (GPCR) of the Frizzled-class (class-F), transduces the Hedgehog signal from the tumour suppressor Patched-1 (PTCH1) to the glioma-associated-oncogene (GLI) transcription factors, which activates the Hedgehog signalling pathway. It has remained unknown how PTCH1 modulates SMO, how SMO is stimulated to form a complex with heterotrimeric G proteins and whether G-protein coupling contributes to the activation of GLI proteins. Here we show that 24,25-epoxycholesterol, which we identify as an endogenous ligand of PTCH1, can stimulate Hedgehog signalling in cells and can trigger G-protein signalling via human SMO in vitro. We present a cryo-electron microscopy structure of human SMO bound to 24(S),25-epoxycholesterol and coupled to a heterotrimeric G protein. The structure reveals a ligand-binding site for 24(S),25-epoxycholesterol in the 7-transmembrane region, as well as a G-coupled activation mechanism of human SMO. Notably, the G protein presents a different arrangement from that of class-A GPCR-G complexes. Our work provides molecular insights into Hedgehog signal transduction and the activation of a class-F GPCR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20190.map.gz emd_20190.map.gz | 78.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20190-v30.xml emd-20190-v30.xml emd-20190.xml emd-20190.xml | 18.1 KB 18.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20190.png emd_20190.png | 58.4 KB | ||
| Filedesc metadata |  emd-20190.cif.gz emd-20190.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20190 http://ftp.pdbj.org/pub/emdb/structures/EMD-20190 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20190 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20190 | HTTPS FTP |
-Related structure data
| Related structure data |  6ot0MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20190.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20190.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Smoothened-Gi complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
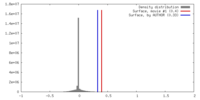
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Smoothened-Gi-Fab complex
+Supramolecule #1: Smoothened-Gi-Fab complex
+Supramolecule #2: Smoothened homolog
+Supramolecule #3: Guanine nucleotide-binding protein G(i) subunit alpha-1, Guanine ...
+Supramolecule #4: Fab light chain, Fab heavy chain
+Macromolecule #1: Smoothened homolog
+Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-1
+Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #5: Fab light chain
+Macromolecule #6: Fab heavy chain
+Macromolecule #7: 17-[3-(3,3-DIMETHYL-OXIRANYL)-1-METHYL-PROPYL]-10,13-DIMETHYL-2,3...
-Experimental details
-Structure determination
 Processing Processing | single particle reconstruction |
|---|---|
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | #0 - Type of model: EMDB MAP #0 - EMDB ID: #1 - Type of model: PDB ENTRY #1 - PDB model - PDB ID: #2 - Type of model: PDB ENTRY #2 - PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.84 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 141100 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller





































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)