[English] 日本語
 Yorodumi
Yorodumi- PDB-3aea: Crystal structure of porcine heart mitochondrial complex II bound... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3aea | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of porcine heart mitochondrial complex II bound with N-(3-Dimethylaminomethyl-phenyl)-2-trifluoromethyl-benzamide | ||||||
 Components Components |
| ||||||
 Keywords Keywords | OXIDOREDUCTASE/OXIDOREDUCTASE INHIBITOR / respiratory complex II / inhibitors / Electron transport / Iron / Iron-sulfur / Metal-binding / Mitochondrion / Mitochondrion inner membrane / Oxidoreductase / Transit peptide / Transport / Tricarboxylic acid cycle / Heme / Transmembrane / FAD-binding protein / OXIDOREDUCTASE-OXIDOREDUCTASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of TCA enzymes and regulation of TCA cycle / Citric acid cycle (TCA cycle) / Oxidoreductases; Acting on the CH-OH group of donors; With a quinone or similar compound as acceptor / succinate metabolic process / respiratory chain complex II (succinate dehydrogenase) / mitochondrial electron transport, succinate to ubiquinone / succinate dehydrogenase (quinone) activity / succinate dehydrogenase / 3 iron, 4 sulfur cluster binding / ubiquinone binding ...Maturation of TCA enzymes and regulation of TCA cycle / Citric acid cycle (TCA cycle) / Oxidoreductases; Acting on the CH-OH group of donors; With a quinone or similar compound as acceptor / succinate metabolic process / respiratory chain complex II (succinate dehydrogenase) / mitochondrial electron transport, succinate to ubiquinone / succinate dehydrogenase (quinone) activity / succinate dehydrogenase / 3 iron, 4 sulfur cluster binding / ubiquinone binding / tricarboxylic acid cycle / protein-membrane adaptor activity / aerobic respiration / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / mitochondrial membrane / flavin adenine dinucleotide binding / nervous system development / 4 iron, 4 sulfur cluster binding / electron transfer activity / mitochondrial inner membrane / heme binding / nucleolus / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 3.39 Å molecular replacement / Resolution: 3.39 Å | ||||||
 Authors Authors | Harada, S. / Sasaki, T. / Shindo, M. / Kido, Y. / Inaoka, D.K. / Omori, J. / Osanai, A. / Sakamoto, K. / Mao, J. / Matsuoka, S. ...Harada, S. / Sasaki, T. / Shindo, M. / Kido, Y. / Inaoka, D.K. / Omori, J. / Osanai, A. / Sakamoto, K. / Mao, J. / Matsuoka, S. / Inoue, M. / Honma, T. / Tanaka, A. / Kita, K. | ||||||
 Citation Citation |  Journal: Int J Mol Sci / Year: 2015 Journal: Int J Mol Sci / Year: 2015Title: Structural Insights into the Molecular Design of Flutolanil Derivatives Targeted for Fumarate Respiration of Parasite Mitochondria Authors: Inaoka, D.K. / Shiba, T. / Sato, D. / Balogun, E.O. / Sasaki, T. / Nagahama, M. / Oda, M. / Matsuoka, S. / Ohmori, J. / Honma, T. / Inoue, M. / Kita, K. / Harada, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3aea.cif.gz 3aea.cif.gz | 452.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3aea.ent.gz pdb3aea.ent.gz | 370.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3aea.json.gz 3aea.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ae/3aea https://data.pdbj.org/pub/pdb/validation_reports/ae/3aea ftp://data.pdbj.org/pub/pdb/validation_reports/ae/3aea ftp://data.pdbj.org/pub/pdb/validation_reports/ae/3aea | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3abvC  3ae7C  3ae9C  4ysxC  4ysyC  4yszC  4yt0C  4ytmC  4ytpC  4yxdC  5c2tC  1zoyS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Succinate dehydrogenase [ubiquinone] ... , 3 types, 3 molecules ABD
| #1: Protein | Mass: 68313.172 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Protein | Mass: 28764.217 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #4: Protein | Mass: 10936.758 Da / Num. of mol.: 1 / Fragment: residues 57-159 / Source method: isolated from a natural source / Source: (natural)  |
-Protein , 1 types, 1 molecules C
| #3: Protein | Mass: 15304.081 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Non-polymers , 8 types, 8 molecules 
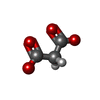




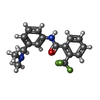







| #5: Chemical | ChemComp-FAD / |
|---|---|
| #6: Chemical | ChemComp-MLI / |
| #7: Chemical | ChemComp-FES / |
| #8: Chemical | ChemComp-SF4 / |
| #9: Chemical | ChemComp-F3S / |
| #10: Chemical | ChemComp-HEM / |
| #11: Chemical | ChemComp-F9A / |
| #12: Chemical | ChemComp-EPH / |
-Details
| Compound details | THESE COMPLEX FORMS MITOCHONDR| Has protein modification | Y | Sequence details | THE SEQUENCE OF CHAIN D IS REFERRED IN REF 2 IN A5GZW8, UNIPROT. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.58 Å3/Da / Density % sol: 65.65 % / Mosaicity: 0.368 ° |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7.2 Details: 25mM HEPES-NAOH, 5% PEG 4000, 200mM Sucrose, 100mM NaCl, 10mM CaCl2, 0.5mM EDTA, 3% 1,6-haxanediol, 0.5% n-decyl-beta-D-maltoside, pH 7.2, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL41XU / Wavelength: 1 Å / Beamline: BL41XU / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Dec 6, 2008 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.39→50 Å / Num. obs: 25146 / % possible obs: 98.3 % / Redundancy: 6.9 % / Rmerge(I) obs: 0.091 / Χ2: 0.939 / Net I/σ(I): 17.333 |
| Reflection shell | Resolution: 3.39→3.51 Å / Redundancy: 6.9 % / Rmerge(I) obs: 0.594 / Mean I/σ(I) obs: 3.18 / Num. unique all: 2338 / Χ2: 1.162 / % possible all: 93.9 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1ZOY Resolution: 3.39→43.77 Å / Cor.coef. Fo:Fc: 0.913 / Cor.coef. Fo:Fc free: 0.878 / WRfactor Rfree: 0.273 / WRfactor Rwork: 0.225 / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.773 / SU B: 62.262 / SU ML: 0.494 / SU R Cruickshank DPI: 0.496 / SU Rfree: 0.599 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.599 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : RESIDUAL ONLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 177.54 Å2 / Biso mean: 113.726 Å2 / Biso min: 20 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.39→43.77 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.386→3.474 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller

































 PDBj
PDBj





















