+Search query
-Structure paper
| Title | Architecture of autoinhibited and active BRAF-MEK1-14-3-3 complexes. |
|---|---|
| Journal, issue, pages | Nature, Vol. 575, Issue 7783, Page 545-550, Year 2019 |
| Publish date | Oct 3, 2019 |
 Authors Authors | Eunyoung Park / Shaun Rawson / Kunhua Li / Byeong-Won Kim / Scott B Ficarro / Gonzalo Gonzalez-Del Pino / Humayun Sharif / Jarrod A Marto / Hyesung Jeon / Michael J Eck /  |
| PubMed Abstract | RAF family kinases are RAS-activated switches that initiate signalling through the MAP kinase cascade to control cellular proliferation, differentiation and survival. RAF activity is tightly ...RAF family kinases are RAS-activated switches that initiate signalling through the MAP kinase cascade to control cellular proliferation, differentiation and survival. RAF activity is tightly regulated and inappropriate activation is a frequent cause of cancer; however, the structural basis for RAF regulation is poorly understood at present. Here we use cryo-electron microscopy to determine autoinhibited and active-state structures of full-length BRAF in complexes with MEK1 and a 14-3-3 dimer. The reconstruction reveals an inactive BRAF-MEK1 complex restrained in a cradle formed by the 14-3-3 dimer, which binds the phosphorylated S365 and S729 sites that flank the BRAF kinase domain. The BRAF cysteine-rich domain occupies a central position that stabilizes this assembly, but the adjacent RAS-binding domain is poorly ordered and peripheral. The 14-3-3 cradle maintains autoinhibition by sequestering the membrane-binding cysteine-rich domain and blocking dimerization of the BRAF kinase domain. In the active state, these inhibitory interactions are released and a single 14-3-3 dimer rearranges to bridge the C-terminal pS729 binding sites of two BRAFs, which drives the formation of an active, back-to-back BRAF dimer. Our structural snapshots provide a foundation for understanding normal RAF regulation and its mutational disruption in cancer and developmental syndromes. |
 External links External links |  Nature / Nature /  PubMed:31581174 / PubMed:31581174 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.59 - 6.8 Å |
| Structure data | EMDB-0541, PDB-6nyb: EMDB-20550, PDB-6q0j: EMDB-20551, PDB-6q0k: EMDB-20552, PDB-6q0t:  PDB-6pp9: |
| Chemicals |  ChemComp-AGS:  ChemComp-ZN:  ChemComp-ADP:  ChemComp-MG: 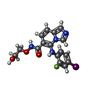 ChemComp-LCJ:  ChemComp-ANP:  ChemComp-CL:  ChemComp-SO4:  ChemComp-GOL:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | TRANSFERASE / BRAF / MEK1 / Transferase/PROTEIN BINDING / Transferase-PROTEIN BINDING complex / SIGNALING PROTEIN/Transferase / SIGNALING PROTEIN-Transferase complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About EMN Papers
About EMN Papers











 homo sapiens (human)
homo sapiens (human) spodoptera exigua (beet armyworm)
spodoptera exigua (beet armyworm)