+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Rat GluN1-2B with Fab 003-102 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Channel / heterotetramer / receptor / antibody / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / positive regulation of Schwann cell migration / pons maturation / sensitization / regulation of cell communication / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors ...cellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / positive regulation of Schwann cell migration / pons maturation / sensitization / regulation of cell communication / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors / response to hydrogen sulfide / auditory behavior / olfactory learning / conditioned taste aversion / dendritic branch / response to other organism / regulation of respiratory gaseous exchange / apical dendrite / regulation of ARF protein signal transduction / fear response / protein localization to postsynaptic membrane / transmitter-gated monoatomic ion channel activity / positive regulation of inhibitory postsynaptic potential / response to methylmercury / suckling behavior / response to manganese ion / response to glycine / interleukin-1 receptor binding / response to carbohydrate / propylene metabolic process / cellular response to dsRNA / cellular response to lipid / response to growth hormone / heterocyclic compound binding / negative regulation of dendritic spine maintenance / positive regulation of glutamate secretion / RAF/MAP kinase cascade / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / Synaptic adhesion-like molecules / voltage-gated monoatomic cation channel activity / response to glycoside / NMDA selective glutamate receptor complex / glutamate binding / ligand-gated sodium channel activity / neurotransmitter receptor complex / response to morphine / regulation of axonogenesis / neuromuscular process / calcium ion transmembrane import into cytosol / regulation of dendrite morphogenesis / protein heterotetramerization / male mating behavior / regulation of synapse assembly / glycine binding / response to amine / regulation of cAMP/PKA signal transduction / receptor clustering / small molecule binding / startle response / parallel fiber to Purkinje cell synapse / positive regulation of reactive oxygen species biosynthetic process / monoatomic cation transmembrane transport / behavioral response to pain / regulation of MAPK cascade / positive regulation of calcium ion transport into cytosol / cellular response to glycine / extracellularly glutamate-gated ion channel activity / regulation of postsynaptic membrane potential / response to magnesium ion / action potential / associative learning / response to electrical stimulus / excitatory synapse / positive regulation of dendritic spine maintenance / social behavior / monoatomic ion channel complex / monoatomic cation transport / regulation of neuronal synaptic plasticity / glutamate receptor binding / Unblocking of NMDA receptors, glutamate binding and activation / positive regulation of excitatory postsynaptic potential / detection of mechanical stimulus involved in sensory perception of pain / long-term memory / response to mechanical stimulus / neuron development / synaptic cleft / phosphatase binding / prepulse inhibition / positive regulation of synaptic transmission, glutamatergic / behavioral fear response / multicellular organismal response to stress / postsynaptic density, intracellular component / monoatomic cation channel activity / response to fungicide / calcium ion homeostasis / glutamate-gated receptor activity / regulation of long-term synaptic depression / cell adhesion molecule binding / regulation of neuron apoptotic process Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.81 Å | |||||||||
 Authors Authors | Michalski K / Furukawa H | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural and functional mechanisms of anti-NMDAR autoimmune encephalitis. Authors: Kevin Michalski / Taha Abdulla / Sam Kleeman / Lars Schmidl / Ricardo Gómez / Noriko Simorowski / Francesca Vallese / Harald Prüss / Manfred Heckmann / Christian Geis / Hiro Furukawa /   Abstract: Autoantibodies against neuronal membrane proteins can manifest in autoimmune encephalitis, inducing seizures, cognitive dysfunction and psychosis. Anti-N-methyl-D-aspartate receptor (NMDAR) ...Autoantibodies against neuronal membrane proteins can manifest in autoimmune encephalitis, inducing seizures, cognitive dysfunction and psychosis. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is the most dominant autoimmune encephalitis; however, insights into how autoantibodies recognize and alter receptor functions remain limited. Here we determined structures of human and rat NMDARs bound to three distinct patient-derived antibodies using single-particle electron cryo-microscopy. These antibodies bind different regions within the amino-terminal domain of the GluN1 subunit. Through electrophysiology, we show that all three autoantibodies acutely and directly reduced NMDAR channel functions in primary neurons. Antibodies show different stoichiometry of binding and antibody-receptor complex formation, which in one antibody, 003-102, also results in reduced synaptic localization of NMDARs. These studies demonstrate mechanisms of diverse epitope recognition and direct channel regulation of anti-NMDAR autoantibodies underlying autoimmune encephalitis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43544.map.gz emd_43544.map.gz | 230 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43544-v30.xml emd-43544-v30.xml emd-43544.xml emd-43544.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43544.png emd_43544.png | 75.1 KB | ||
| Filedesc metadata |  emd-43544.cif.gz emd-43544.cif.gz | 7.2 KB | ||
| Others |  emd_43544_half_map_1.map.gz emd_43544_half_map_1.map.gz emd_43544_half_map_2.map.gz emd_43544_half_map_2.map.gz | 226.5 MB 226.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43544 http://ftp.pdbj.org/pub/emdb/structures/EMD-43544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43544 | HTTPS FTP |
-Validation report
| Summary document |  emd_43544_validation.pdf.gz emd_43544_validation.pdf.gz | 885.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43544_full_validation.pdf.gz emd_43544_full_validation.pdf.gz | 885.3 KB | Display | |
| Data in XML |  emd_43544_validation.xml.gz emd_43544_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  emd_43544_validation.cif.gz emd_43544_validation.cif.gz | 18.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43544 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43544 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43544 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43544 | HTTPS FTP |
-Related structure data
| Related structure data |  8vuyMC  8vuhC  8vujC  8vulC  8vunC  8vuqC  8vurC  8vusC  8vutC  8vuuC  8vuvC  8vvhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43544.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43544.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.856 Å | ||||||||||||||||||||||||||||||||||||
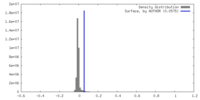
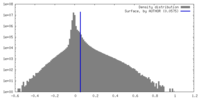
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_43544_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
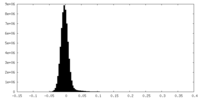
| Density Histograms |
-Half map: #2
| File | emd_43544_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
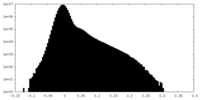
| Density Histograms |
- Sample components
Sample components
-Entire : Rat GluN1-2B with Fab 003-102
| Entire | Name: Rat GluN1-2B with Fab 003-102 |
|---|---|
| Components |
|
-Supramolecule #1: Rat GluN1-2B with Fab 003-102
| Supramolecule | Name: Rat GluN1-2B with Fab 003-102 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glutamate receptor ionotropic, NMDA 1
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 91.944094 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KIVNIGAVLS TRKHEQMFRE AVNQANKRHG SWKIQLQATS VTHKPNAIQM ALSVCEDLIS SQVYAILVSH PPTPNDHFTP TPVSYTAGF YRIPVLGLTT RMSIYSDKSI HLSFLRTVPP YSHQSSVWFE MMRVYNWNHI ILLVSDDHEG RAAQKRLETL L EERESKAE ...String: KIVNIGAVLS TRKHEQMFRE AVNQANKRHG SWKIQLQATS VTHKPNAIQM ALSVCEDLIS SQVYAILVSH PPTPNDHFTP TPVSYTAGF YRIPVLGLTT RMSIYSDKSI HLSFLRTVPP YSHQSSVWFE MMRVYNWNHI ILLVSDDHEG RAAQKRLETL L EERESKAE KVLQFDPGTK NVTALLMEAR ELEARVIILS ASEDDAATVY RAAAMLDMTG SGYVWLVGER EISGNALRYA PD GIIGLQL INGKNESAHI SDAVGVVAQA VHELLEKENI TDPPRGCVGN TNIWKTGPLF KRVLMSSKYA DGVTGRVEFN EDG DRKFAQ YSIMNLQNRK LVQVGIYNGT HVIPNDRKII WPGGETEKPR GYQMSTRLKI VTIHQEPFVY VKPTMSDGTC KEEF TVNGD PVKKVICTGP NDTSPGSPRH TVPQCCYGFC IDLLIKLART MQFTYEVHLV ADGKFGTQER VQNSNKKEWN GMMGE LLSG QADMIVAPLT INNERAQYIE FSKPFKYQGL TILVKKEIPR STLDSFMNPF QSTLWLLVGL SVHVVAVMLY LLDRFS PFG RFKVNSEEEE EDALTLSSAM WFSWGVLLNS GIGEGAPRSF SARILGMVWA GFAMIIVASY TANLAAFLVL DRPEERI TG INDPRLRNPS DKFIYATVKQ SSVDIYFRRQ VELSTMYRHM EKHNYESAAE AIQAVRDNKL HAFIWDSAVL EFEASQKC D LVTTGELFFR SGFGIGMRKD SPWKQQVSLS ILKSHENGFM EDLDKTWVRY QECDSRSNAP ATLTFENMAG VFMIVAGGI VAGIFLIFIE IAYKSRA UniProtKB: Glutamate receptor ionotropic, NMDA 1 |
-Macromolecule #2: Glutamate receptor ionotropic, NMDA 2B
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 2B / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 91.269125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SIGIAVILVG TSDEVAIKDA HEKDDFHHLS VVPRVELVAM NETDPKSIIT RICDLMSDRK IQGVVFADDT DQEAIAQILD FISAQTLTP ILGIHGGSSM IMADKDESSM FFQFGPSIEQ QASVMLNIME EYDWYIFSIV TTYFPGYQDF VNKIRSTIEN S FVGWELEE ...String: SIGIAVILVG TSDEVAIKDA HEKDDFHHLS VVPRVELVAM NETDPKSIIT RICDLMSDRK IQGVVFADDT DQEAIAQILD FISAQTLTP ILGIHGGSSM IMADKDESSM FFQFGPSIEQ QASVMLNIME EYDWYIFSIV TTYFPGYQDF VNKIRSTIEN S FVGWELEE VLLLDMSLDD GDSKIQNQLK KLQSPIILLY CTKEEATYIF EVANSVGLTG YGYTWIVPSL VAGDTDTVPS EF PTGLISV SYDEWDYGLP ARVRDGIAII TTAASDMLSE HSFIPEPKSS CYNTHEKRIY QSNMLNRYLI NVTFEGRDLS FSE EGYQMH PKLVIILLNK ERKWERVGKW KDKSLQMKYY VWPRMCPETE EQEDDHLSIV TLEEAPFVIV ESVDPLSGTC MRNT VPCEK RIISENKTDE EPGYIKKCCK GFCIDILKKI SKSVKFTYDL YLVTNGKHGK KINGTWNGMI GEVVMKRAYM AVGSL TINE ERSEVVDFSV PFIETGISVM VSRSNGTVSP SAFLEPFSAD VWVMMFVMLL IVSAVAVFVF EYFSPVGYNR CLADGR EPG GPSFTIGKAI WLLWGLVFNN SVPVQNPKGT TSKIMVSVWA FFAVIFLASY TANLAAFMIQ EEYVDQVSGL SDKKFQR PN DFSPPFRFGT VPNGSTERNI RNNYAEMHAY MGKFNQRGVD DALLSLKTGK LDAFIYDAAV LNYMAGRDEG CKLVTIGS G KVFASTGYGI AIQKDSGWKR QVDLAILQLF GDGEMEELEA LWLTGICHNE KNEVMSSQLD IDNMAGVFYM LGAAMALSL ITFISEHLFY WQ UniProtKB: Glutamate receptor ionotropic, NMDA 2B |
-Macromolecule #3: 003-102 Heavy
| Macromolecule | Name: 003-102 Heavy / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.477862 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: LQLQESGPGL VKPSQTLSLT CTVSGGSISS SNWWSWVRQP PGKGLEWIGE IYHSGNTNYN PSLKSRVTVS VDKSKNQFSL KLTSVTAAD TAVYYCARDV SGGVNWFDPW GQGTLV |
-Macromolecule #4: 003-102 Light
| Macromolecule | Name: 003-102 Light / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.582475 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NFMLTQPHSV SESPGKTVTI SCTRSSGSIA SNYVQWYQQR PGSAPTTVIY EDNQRPSGVP DRFSGSIDSS SNSASLTISG LKTEDEADY YCQSYDSSTV VFGGGTKLT |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)