[English] 日本語
 Yorodumi
Yorodumi- EMDB-42427: CryoEM structure of beta-2-adrenergic receptor in complex with GT... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of beta-2-adrenergic receptor in complex with GTP-bound Gs heterotrimer (Class T) | |||||||||
 Map data Map data | structure of beta-2-adrenergic receptor in complex with GTP-bound Gs heterotrimer (Class T) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / Adrenergic / Receptor / G protein / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / AMPA selective glutamate receptor signaling pathway / norepinephrine binding / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / Adrenoceptors / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction ...positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / AMPA selective glutamate receptor signaling pathway / norepinephrine binding / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / Adrenoceptors / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction / positive regulation of lipophagy / negative regulation of multicellular organism growth / negative regulation of G protein-coupled receptor signaling pathway / response to psychosocial stress / adrenergic receptor signaling pathway / endosome to lysosome transport / diet induced thermogenesis / neuronal dense core vesicle / positive regulation of cAMP/PKA signal transduction / PKA activation in glucagon signalling / adenylate cyclase binding / hair follicle placode formation / smooth muscle contraction / developmental growth / D1 dopamine receptor binding / intracellular transport / renal water homeostasis / Hedgehog 'off' state / bone resorption / positive regulation of bone mineralization / potassium channel regulator activity / brown fat cell differentiation / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / regulation of sodium ion transport / cellular response to glucagon stimulus / adenylate cyclase activator activity / regulation of insulin secretion / response to cold / receptor-mediated endocytosis / trans-Golgi network membrane / negative regulation of inflammatory response to antigenic stimulus / clathrin-coated endocytic vesicle membrane / bone development / G-protein beta/gamma-subunit complex binding / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / adenylate cyclase-activating G protein-coupled receptor signaling pathway / platelet aggregation / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / cognition / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / cellular response to amyloid-beta / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / sensory perception of taste / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / sensory perception of smell / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / Cargo recognition for clathrin-mediated endocytosis / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / Clathrin-mediated endocytosis / G protein activity / GTPase binding / positive regulation of cold-induced thermogenesis / amyloid-beta binding / retina development in camera-type eye / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / fibroblast proliferation / G alpha (i) signalling events Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Papasergi-Scott MM / Skiniotis G | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Time-resolved cryo-EM of G-protein activation by a GPCR. Authors: Makaía M Papasergi-Scott / Guillermo Pérez-Hernández / Hossein Batebi / Yang Gao / Gözde Eskici / Alpay B Seven / Ouliana Panova / Daniel Hilger / Marina Casiraghi / Feng He / Luis Maul ...Authors: Makaía M Papasergi-Scott / Guillermo Pérez-Hernández / Hossein Batebi / Yang Gao / Gözde Eskici / Alpay B Seven / Ouliana Panova / Daniel Hilger / Marina Casiraghi / Feng He / Luis Maul / Peter Gmeiner / Brian K Kobilka / Peter W Hildebrand / Georgios Skiniotis /    Abstract: G-protein-coupled receptors (GPCRs) activate heterotrimeric G proteins by stimulating guanine nucleotide exchange in the Gα subunit. To visualize this mechanism, we developed a time-resolved cryo-EM ...G-protein-coupled receptors (GPCRs) activate heterotrimeric G proteins by stimulating guanine nucleotide exchange in the Gα subunit. To visualize this mechanism, we developed a time-resolved cryo-EM approach that examines the progression of ensembles of pre-steady-state intermediates of a GPCR-G-protein complex. By monitoring the transitions of the stimulatory G protein in complex with the β-adrenergic receptor at short sequential time points after GTP addition, we identified the conformational trajectory underlying G-protein activation and functional dissociation from the receptor. Twenty structures generated from sequential overlapping particle subsets along this trajectory, compared to control structures, provide a high-resolution description of the order of main events driving G-protein activation in response to GTP binding. Structural changes propagate from the nucleotide-binding pocket and extend through the GTPase domain, enacting alterations to Gα switch regions and the α5 helix that weaken the G-protein-receptor interface. Molecular dynamics simulations with late structures in the cryo-EM trajectory support that enhanced ordering of GTP on closure of the α-helical domain against the nucleotide-bound Ras-homology domain correlates with α5 helix destabilization and eventual dissociation of the G protein from the GPCR. These findings also highlight the potential of time-resolved cryo-EM as a tool for mechanistic dissection of GPCR signalling events. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42427.map.gz emd_42427.map.gz | 127 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42427-v30.xml emd-42427-v30.xml emd-42427.xml emd-42427.xml | 25.4 KB 25.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42427_fsc.xml emd_42427_fsc.xml | 10.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_42427.png emd_42427.png | 88.6 KB | ||
| Masks |  emd_42427_msk_1.map emd_42427_msk_1.map | 134.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42427.cif.gz emd-42427.cif.gz | 7.8 KB | ||
| Others |  emd_42427_additional_1.map.gz emd_42427_additional_1.map.gz emd_42427_half_map_1.map.gz emd_42427_half_map_1.map.gz emd_42427_half_map_2.map.gz emd_42427_half_map_2.map.gz | 67.1 MB 125 MB 125 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42427 http://ftp.pdbj.org/pub/emdb/structures/EMD-42427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42427 | HTTPS FTP |
-Validation report
| Summary document |  emd_42427_validation.pdf.gz emd_42427_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42427_full_validation.pdf.gz emd_42427_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_42427_validation.xml.gz emd_42427_validation.xml.gz | 19.3 KB | Display | |
| Data in CIF |  emd_42427_validation.cif.gz emd_42427_validation.cif.gz | 24.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42427 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42427 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42427 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42427 | HTTPS FTP |
-Related structure data
| Related structure data |  8uo4MC  8gdzC  8ge1C  8ge2C  8ge3C  8ge4C  8ge5C  8ge6C  8ge7C  8ge8C  8ge9C  8geaC  8gebC  8gecC  8gedC  8geeC  8gefC  8gegC  8gehC  8geiC  8gejC  8gfvC  8gfwC  8gfxC  8gfyC  8gfzC  8gg0C  8gg1C  8gg2C  8gg3C  8gg4C  8gg5C  8gg6C  8gg7C  8gg8C  8gg9C  8ggaC  8ggbC  8ggcC  8ggeC  8ggfC  8ggiC  8ggjC  8ggkC  8gglC  8ggmC  8ggnC  8ggoC  8ggpC  8ggqC  8ggrC  8ggsC  8ggtC  8gguC  8ggvC  8ggwC  8ggxC  8ggyC  8ggzC  8gh0C  8gh1C  8unlC  8unmC  8unnC  8unoC  8unpC  8unqC  8unrC  8unsC  8untC  8unuC  8unvC  8unwC  8unxC  8unyC  8unzC  8uo0C  8uo1C  8uo2C  8uo3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42427.map.gz / Format: CCP4 / Size: 134.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42427.map.gz / Format: CCP4 / Size: 134.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | structure of beta-2-adrenergic receptor in complex with GTP-bound Gs heterotrimer (Class T) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8677 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42427_msk_1.map emd_42427_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
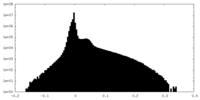
| Density Histograms |
-Additional map: #1
| File | emd_42427_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_42427_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_42427_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of beta-2 adrenergic receptor and Gs heterotrimer with GTP
| Entire | Name: Complex of beta-2 adrenergic receptor and Gs heterotrimer with GTP |
|---|---|
| Components |
|
-Supramolecule #1: Complex of beta-2 adrenergic receptor and Gs heterotrimer with GTP
| Supramolecule | Name: Complex of beta-2 adrenergic receptor and Gs heterotrimer with GTP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: Combined datasets of complex plunge frozen at 5 sec, 10 sec, or 17 sec after GTP addition to the sample. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.32616 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG DSEKATKVQD IKNNLKEAI ETIVAAMSNL VPPVELANPE NQFRVDYILS VMNVPDFDFP PEFYEHAKAL WEDEGVRACY ERSNEYQLID C AQYFLDKI ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG DSEKATKVQD IKNNLKEAI ETIVAAMSNL VPPVELANPE NQFRVDYILS VMNVPDFDFP PEFYEHAKAL WEDEGVRACY ERSNEYQLID C AQYFLDKI DVIKQADYVP SDQDLLRCRV LTSGIFETKF QVDKVNFHMF DVGGQRDERR KWIQCFNDVT AIIFVVASSS YN MVIREDN QTNRLQEALN LFKSIWNNRW LRTISVILFL NKQDLLAEKV LAGKSKIEDY FPEFARYTTP EDATPEPGED PRV TRAKYF IRDEFLRIST ASGDGRHYCY PHFTCAVDTE NIRRVFNDCR DIIQRMHLRQ YELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.573988 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GSSGSELDQL RQEAEQLKNQ IRDARKACAD ATLSQITNNI DPVGRIQMRT RRTLRGHLAK IYAMHWGTDS RLLVSASQDG KLIIWDSYT TNKVHAIPLR SSWVMTCAYA PSGNYVACGG LDNICSIYNL KTREGNVRVS RELAGHTGYL SCCRFLDDNQ I VTSSGDTT ...String: GSSGSELDQL RQEAEQLKNQ IRDARKACAD ATLSQITNNI DPVGRIQMRT RRTLRGHLAK IYAMHWGTDS RLLVSASQDG KLIIWDSYT TNKVHAIPLR SSWVMTCAYA PSGNYVACGG LDNICSIYNL KTREGNVRVS RELAGHTGYL SCCRFLDDNQ I VTSSGDTT CALWDIETGQ QTTTFTGHTG DVMSLSLAPD TRLFVSGACD ASAKLWDVRE GMCRQTFTGH ESDINAICFF PN GNAFATG SDDATCRLFD LRADQELMTY SHDNIICGIT SVSFSKSGRL LLAGYDDFNC NVWDALKADR AGVLAGHDNR VSC LGVTDD GMAVATGSWD SFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Beta-2 adrenergic receptor
| Macromolecule | Name: Beta-2 adrenergic receptor / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.767242 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDAMGQPGN GSAFLLAPNR SHAPDHDVEN LYFQGTQQRD EVWVVGMGIV MSLIVLAIVF GNVLVITAI AKFERLQTVT NYFITSLACA DLVMGLAVVP FGAAHILTKT WTFGNFWCEF WTSIDVLCVT ASIETLCVIA V DRYFAITS ...String: MKTIIALSYI FCLVFADYKD DDDAMGQPGN GSAFLLAPNR SHAPDHDVEN LYFQGTQQRD EVWVVGMGIV MSLIVLAIVF GNVLVITAI AKFERLQTVT NYFITSLACA DLVMGLAVVP FGAAHILTKT WTFGNFWCEF WTSIDVLCVT ASIETLCVIA V DRYFAITS PFKYQSLLTK NKARVIILMV WIVSGLTSFL PIQMHWYRAT HQEAINCYAE ETCCDFFTNQ AYAIASSIVS FY VPLVIMV FVYSRVFQEA KRQLQKIDKS EGRFHVQNLS QVEQDGRTGH GLRRSSKFCL KEHKALKTLG IIMGTFTLCW LPF FIVNIV HVIQDNLIRK EVYILLNWIG YVNSGFNPLI YCRSPDFRIA FQELLCLRRS SLKAYGNGYS SNGNTGEQSG LEVL FQGPY HVEQEKENKL LAEDLPGTED FVGHQGTVPS DNIDSQGRNA STNDSLLETS QVAPA UniProtKB: Beta-2 adrenergic receptor |
-Macromolecule #5: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #7: (5R,6R)-6-(methylamino)-5,6,7,8-tetrahydronaphthalene-1,2,5-triol
| Macromolecule | Name: (5R,6R)-6-(methylamino)-5,6,7,8-tetrahydronaphthalene-1,2,5-triol type: ligand / ID: 7 / Number of copies: 1 / Formula: G1I |
|---|---|
| Molecular weight | Theoretical: 209.242 Da |
| Chemical component information |  ChemComp-G1I: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: GTP was added just prior to freezing at 5 sec, 10 sec, or 17 sec before plunging. |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: GTP was added just prior to freezing at 5 sec, 10 sec, or 17 sec before plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 Details: The cryoEM map is the result of combining multiple datasets: cryo-EM imaging of the beta-2AR-Gs + GTP (5 sec) complex was performed on a Titan Krios electron microscope equipped with a K3 ...Details: The cryoEM map is the result of combining multiple datasets: cryo-EM imaging of the beta-2AR-Gs + GTP (5 sec) complex was performed on a Titan Krios electron microscope equipped with a K3 Summit direct electron detector (Gatan). The microscope was operated at 300 kV accelerating voltage, with a nominal magnification of 105,000x in counting mode resulting in a magnified pixel size of 0.8677 Angstrom. A total exposure of 60.48 electrons/ Angstrom^2 over 63 frames with defocus ranging from -1.0 - -2.0 micrometers was used. Cryo-EM imaging of beta-2AR-Gs + GTP (10 sec) complex was performed on four separate grids over three collection sessions. The microscope was operated at 300 kV accelerating voltage, with a magnification at the camera of 58,679x in counting mode resulting in a magnified pixel size of 0.8521 Angstrom. For the first and second grids, movies were obtained at an exposure rate of 21.13 electrons/Angstrum^2/sec with defocus ranging from -0.4 - -2.0 micrometers. The total exposure time was 2.717 sec over 77 frames per movie stack. For an additional collection of the first grid, movies were obtained at an exposure rate of 20.95 electrons/ Angstrum^2/sec with defocus ranging from -0.4 -2.0 micrometers. The total exposure time was 2.717 sec over 77 frames per movie stack. For the third and fourth grids, movies were obtained at an exposure rate of 30.71 electrons/ Angstrum^2/sec with defocus ranging from -0.5 - -1.6 micrometers. The total exposure time was 2.008 sec over 79 frames per movie stack. Cryo-EM imaging of beta-2AR-Gs + GTP (17 sec) was performed on a Titan Krios equipped with a post-column energy filter, with a magnification of 105,000x in counting mode resulting in a magnified pixel size of 0.8677 Angstrom. Movies were obtained at an exposure rate of 32.46 electrons/Angstrum^2/sec with defocus ranging from -0.4 - -0.9 micrometers. The total exposure time was 1.999 sec over 79 frames per movie stack. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.4 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























































































































































































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)