[English] 日本語
 Yorodumi
Yorodumi- EMDB-25786: Refined capsid structure of human adenovirus D26 at 3.4 A resolution -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25786 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Refined capsid structure of human adenovirus D26 at 3.4 A resolution | |||||||||
 Map data Map data | Cropped version of the original cryo-EM map 928x928x928 pixels. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Human Adenovirus D26 HAdV-D26 Ad26 Hexon Penton base IX / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationhexon binding / viral capsid, decoration / T=25 icosahedral viral capsid / lysis of host organelle involved in viral entry into host cell / viral procapsid / microtubule-dependent intracellular transport of viral material towards nucleus / adhesion receptor-mediated virion attachment to host cell / viral release from host cell / viral capsid / host cell ...hexon binding / viral capsid, decoration / T=25 icosahedral viral capsid / lysis of host organelle involved in viral entry into host cell / viral procapsid / microtubule-dependent intracellular transport of viral material towards nucleus / adhesion receptor-mediated virion attachment to host cell / viral release from host cell / viral capsid / host cell / host cell cytoplasm / cell adhesion / endocytosis involved in viral entry into host cell / symbiont entry into host cell / host cell nucleus / virion attachment to host cell / structural molecule activity Similarity search - Function | |||||||||
| Biological species |  Human adenovirus 26 Human adenovirus 26 | |||||||||
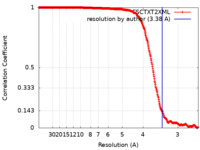
| Method | single particle reconstruction / cryo EM / Resolution: 3.38 Å | |||||||||
 Authors Authors | Reddy VS / Yu X | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2017 Journal: Sci Adv / Year: 2017Title: Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Authors: Xiaodi Yu / David Veesler / Melody G Campbell / Mary E Barry / Francisco J Asturias / Michael A Barry / Vijay S Reddy /  Abstract: Human adenoviruses (HAdVs) cause acute respiratory, ocular, and gastroenteric diseases and are also frequently used as gene and vaccine delivery vectors. Unlike the archetype human adenovirus C5 ...Human adenoviruses (HAdVs) cause acute respiratory, ocular, and gastroenteric diseases and are also frequently used as gene and vaccine delivery vectors. Unlike the archetype human adenovirus C5 (HAdV-C5), human adenovirus D26 (HAdV-D26) belongs to species-D HAdVs, which target different cellular receptors, and is differentially recognized by immune surveillance mechanisms. HAdV-D26 is being championed as a lower seroprevalent vaccine and oncolytic vector in preclinical and human clinical studies. To understand the molecular basis for their distinct biological properties and independently validate the structures of minor proteins, we determined the first structure of species-D HAdV at 3.7 Å resolution by cryo-electron microscopy. All the hexon hypervariable regions (HVRs), including HVR1, have been identified and exhibit a distinct organization compared to those of HAdV-C5. Despite the differences in the arrangement of helices in the coiled-coil structures, protein IX molecules form a continuous hexagonal network on the capsid exterior. In addition to the structurally conserved region (3 to 300) of IIIa, we identified an extra helical domain comprising residues 314 to 390 that further stabilizes the vertex region. Multiple (two to three) copies of the cleaved amino-terminal fragment of protein VI (pVIn) are observed in each hexon cavity, suggesting that there could be ≥480 copies of VI present in HAdV-D26. In addition, a localized asymmetric reconstruction of the vertex region provides new details of the three-pronged "claw hold" of the trimeric fiber and its interactions with the penton base. These observations resolve the previous conflicting assignments of the minor proteins and suggest the likely conservation of their organization across different HAdVs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25786.map.gz emd_25786.map.gz | 2 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25786-v30.xml emd-25786-v30.xml emd-25786.xml emd-25786.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25786_fsc.xml emd_25786_fsc.xml | 33.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_25786.png emd_25786.png | 242.2 KB | ||
| Filedesc metadata |  emd-25786.cif.gz emd-25786.cif.gz | 7.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25786 http://ftp.pdbj.org/pub/emdb/structures/EMD-25786 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25786 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25786 | HTTPS FTP |
-Validation report
| Summary document |  emd_25786_validation.pdf.gz emd_25786_validation.pdf.gz | 815.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25786_full_validation.pdf.gz emd_25786_full_validation.pdf.gz | 815.5 KB | Display | |
| Data in XML |  emd_25786_validation.xml.gz emd_25786_validation.xml.gz | 22.6 KB | Display | |
| Data in CIF |  emd_25786_validation.cif.gz emd_25786_validation.cif.gz | 32.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25786 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25786 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25786 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25786 | HTTPS FTP |
-Related structure data
| Related structure data |  7tauMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25786.map.gz / Format: CCP4 / Size: 2.1 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25786.map.gz / Format: CCP4 / Size: 2.1 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cropped version of the original cryo-EM map 928x928x928 pixels. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human adenovirus 26
| Entire | Name:  Human adenovirus 26 Human adenovirus 26 |
|---|---|
| Components |
|
-Supramolecule #1: Human adenovirus 26
| Supramolecule | Name: Human adenovirus 26 / type: tissue / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Adenovirus |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
-Macromolecule #1: Hexon protein
| Macromolecule | Name: Hexon protein / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 107.218703 KDa |
| Sequence | String: MATPSMMPQW AYMHIAGQDA SEYLSPGLVQ FARATDTYFS LGNKFRNPTV APTHDVTTDR SQRLTLRFVP VDREATTYLY KARFTLAVG DNRVLDMAST YFDIRGVLDR GPSFKPYSGT AYNSLAPKGA PNPSQWETKE KQGTTGGVQQ EKDVTKTFGV A ATGGINIT ...String: MATPSMMPQW AYMHIAGQDA SEYLSPGLVQ FARATDTYFS LGNKFRNPTV APTHDVTTDR SQRLTLRFVP VDREATTYLY KARFTLAVG DNRVLDMAST YFDIRGVLDR GPSFKPYSGT AYNSLAPKGA PNPSQWETKE KQGTTGGVQQ EKDVTKTFGV A ATGGINIT NQGLLLGTDE TAENGKKDIY ADKTFQPEPQ VGEENWQENE AFYGGRALKK DTKMKPCYGS FARPTNEKGG QA KFKPVNE GEQPKDLDID FAYFDVPGGS PPAGGSGEEY KADIILYTEN VNLETPDTHV VYKPGTSDNS SEINLVQQSM PNR PNYIGF RDNFVGLMYY NSTGNMGVLA GQASQLNAVV DLQDRNTELS YQLLLDSLGD RTRYFSMWNS AVDSYDPDVR IIEN HGVED ELPNYCFPLN GTGTNSTYQG VKITNGNDGA EESEWEKDDA ISRQNQICKG NVYAMEINLQ ANLWKSFLYS NVALY LPDS YKYTPANVKL PANTNTYEYM NGRVVAPSLV DAYINIGARW SLDPMDNVNP FNHPRNAGLR YRSMLLGNGR YVPFHI QVP QKFFAIKNLL LLPGSYTYEW NFRKDVNMIL QSSLGNDLRV DGASVRFDSV NLYATFFPMA HNTASTLEAM LRNDTHD QS FNDYLSAANM LYPIPAKATN VPISIPSRNW AAFRGWSFTR LKTKETPSLG SGFDPYFVYS GSIPYLDGTF YLNHTFKK V SIMFDSSVSW PGNDRLLTPN EFEIKRSVDG EGYNVAQCNM TKDWFLVQML SHYNIGYQGF HVPEGYKDRM YSFFRNFQP MSRQVVDEIN YKDYKAVTLP FQHNNSGFTG YLAPTMRQGQ PYPANFPYPL IGQTAVPSVT QKKFLCDRVM WRIPFSSNFM SMGALTDLG QNMLYANSAH ALDMTFEVDP MDEPTLLYLL FEVFDVVRVH QPHRGVIEAV YLRTPFSAGN ATT UniProtKB: Hexon protein |
-Macromolecule #2: Penton protein
| Macromolecule | Name: Penton protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 58.647703 KDa |
| Sequence | String: MRRAVVSSSP PPSYESVMAQ ATLEVPFVPP RYMAPTEGRN SIRYSELAPQ YDTTRVYLVD NKSADIASLN YQNDHSNFLT TVVQNNDFT PAEASTQTIN FDERSRWGGD LKTILHTNMP NVNEYMFTSK FKARVMVSRK HPEGVVETDL SQDKLEYEWF E FTLPEGNF ...String: MRRAVVSSSP PPSYESVMAQ ATLEVPFVPP RYMAPTEGRN SIRYSELAPQ YDTTRVYLVD NKSADIASLN YQNDHSNFLT TVVQNNDFT PAEASTQTIN FDERSRWGGD LKTILHTNMP NVNEYMFTSK FKARVMVSRK HPEGVVETDL SQDKLEYEWF E FTLPEGNF SETMTIDLMN NAILENYLQV GRQNGVLESD IGVKFDSRNF KLGWDPVTKL VMPGVYTYEA FHPDVVLLPG CG VDFTESR LSNLLGIRKK QPFQEGFRIM YEDLEGGNIP ALLDVPKYLE SKKKVEDETK NAAAATADTT TRGDTFATPA QET AADKKV EVLPIEKDES GRSYNLIQGT HDTLYRSWYL SYTYGDPEKG VQSWTLLTTP DVTCGAEQVY WSLPDLMQDP VTFR STQQV SNYPVVGAEL MPFRAKSFYN DLAVYSQLIR SYTSLTHVFN RFPDNQILCR PPAPTITTVS ENVPALTDHG TLPLR SSIR GVQRVTVTDA RRRTCPYVYK ALGIVAPRVL SSRTF UniProtKB: Penton protein |
-Macromolecule #3: Fiber
| Macromolecule | Name: Fiber / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 41.039184 KDa |
| Sequence | String: MAKRLRVEDD FNPVYPYGYA RNQNIPFLTP PFVSSDGFKN FPPGVLSLKL ADPITINNGD VSLKVGGGLA VEQQTGNLSV NPDAPLQVA SDKLQLALAP PFEVRDGKLA LKAGNGLKVL DNSITGLTGL LNTLVVLTGR GIGTEELKND DGVTNKGVGL R VRLGDDGG ...String: MAKRLRVEDD FNPVYPYGYA RNQNIPFLTP PFVSSDGFKN FPPGVLSLKL ADPITINNGD VSLKVGGGLA VEQQTGNLSV NPDAPLQVA SDKLQLALAP PFEVRDGKLA LKAGNGLKVL DNSITGLTGL LNTLVVLTGR GIGTEELKND DGVTNKGVGL R VRLGDDGG LTFDKKGDLV AWNKKDDRRT LWTTPDTSPN CKMSTEKDSK LTLTLTKCGS QVLGNVSLLA VTGEYHQMTA TT KKDVKIS LLFDENGILL PSSSLSKDYW NYRSDDSIVS QKYNNAVPFM PNLTAYPKPS AQNAKNYSRT KIISNVYLGA LTY QPVIIT IAFNQETENG CAYSITFTFT WQKDYSAQQF DVTSFTFSYL TQENKDKD UniProtKB: Fiber |
-Macromolecule #4: Pre-hexon-linking protein IIIa
| Macromolecule | Name: Pre-hexon-linking protein IIIa / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 62.201371 KDa |
| Sequence | String: MSQQAPDPAI RAALQSQPSG LASDDWEAAM QRIMALTTRN PESFRQQPQA NRLSAILEAV VPSRTNPTHE KVLAIVNALA ENKAIRPDE AGLVYNALLE RVGRYNSTNV QSNLDRLVTD VREAVAQRER FKNEGLGSLV ALNAFLATQP ANVPRGQDDY T NFISALRL ...String: MSQQAPDPAI RAALQSQPSG LASDDWEAAM QRIMALTTRN PESFRQQPQA NRLSAILEAV VPSRTNPTHE KVLAIVNALA ENKAIRPDE AGLVYNALLE RVGRYNSTNV QSNLDRLVTD VREAVAQRER FKNEGLGSLV ALNAFLATQP ANVPRGQDDY T NFISALRL MVTEVPQSEV YQSGPDYFFQ TSRQGLQTVN LSQAFKNLRG LWGVQAPVGD RSTVSSLLTP NSRLLLLLIA PF TDSGSVN RNSYLGHLLT LYREAIGQAQ VDEQTFQEIT SVSRALGQND TDSLRATLNF LLTNRQQKIP AQYALSAEEE RIL RYVQQS VGLFLMQEGA TPSAALDMTA RNMEPSMYAA NRPFINKLMD YLHRAAAMNT DYFTNAILNP HWLPPPGFYT GEYD MPDPN DGFLWDDVDS AVFSPTFQKR QEAPPSEGAV GRSPFPSLGS LHSLPGSVNS GRVSRPRLLG EDEYLNDSLL QPPRA KNAM ANNGIESLVD KLNRWKTYAQ DHRDAPAPRR QRHDRQRGLV WDDEDSADDS SVLDLGGSGG VNPFAHLQPK LGRRMF UniProtKB: Pre-hexon-linking protein IIIa |
-Macromolecule #5: PIX
| Macromolecule | Name: PIX / type: protein_or_peptide / ID: 5 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 13.800377 KDa |
| Sequence | String: MNGTGGAFEG GLFSPYLTTR LPGWAGVRQN VMGSTVDGRP VLPANSSTMT YATVGNSSLD STAAAAAAAA AMTATRLASS YMPSSGSSP SVPSSIIAEE KLLALLAELE ALSRQLAALT QQVSELREQQ QQQNK UniProtKB: Hexon-interlacing protein |
-Macromolecule #6: PVIII
| Macromolecule | Name: PVIII / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 24.633643 KDa |
| Sequence | String: MSKEIPTPYM WSYQPQMGLA AGASQDYSTR MNWLSAGPSM ISRVNGVRSH RNQILLEQAA VTSTPRAKLN PRNWPSTLVY QEIPGPTTV LLPRDALAEV RMTNSGVQLA GGASRCPLRP QSGIKTLVIR GRGTQLNDEL VSSSIGLRPD GVFQLAGAGR S SFTPNQAY ...String: MSKEIPTPYM WSYQPQMGLA AGASQDYSTR MNWLSAGPSM ISRVNGVRSH RNQILLEQAA VTSTPRAKLN PRNWPSTLVY QEIPGPTTV LLPRDALAEV RMTNSGVQLA GGASRCPLRP QSGIKTLVIR GRGTQLNDEL VSSSIGLRPD GVFQLAGAGR S SFTPNQAY LTLQSSSSEP RSGGIGTLQF VEEFVPSVYF NPFSGSPGLY PDEFIPNFDA VREAVDGYD UniProtKB: Pre-hexon-linking protein VIII |
-Macromolecule #7: Pre-protein VI
| Macromolecule | Name: Pre-protein VI / type: protein_or_peptide / ID: 7 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 25.546086 KDa |
| Sequence | String: MEDINFASLA PRHGTRPFMG TWNEIGTSQL NGGAFNWSSV WSGLKNFGST LRTYGNKAWN SSTGQLLREK LKDQNFQQKV VDGLASGIN GVVDIANQAV QREINSRLDP RPPTVVEMED ATLPPPKGEK RPRPDAEETI LQVDEPPSYE EAVKAGMPTT R IIAPLATG ...String: MEDINFASLA PRHGTRPFMG TWNEIGTSQL NGGAFNWSSV WSGLKNFGST LRTYGNKAWN SSTGQLLREK LKDQNFQQKV VDGLASGIN GVVDIANQAV QREINSRLDP RPPTVVEMED ATLPPPKGEK RPRPDAEETI LQVDEPPSYE EAVKAGMPTT R IIAPLATG VMKPATLDLP PPPAPAPPKA TPVVQAPPVA TAVRRVPARR QAQNWQSTLH SIVGLGVKSL KRRRCY UniProtKB: Pre-protein VI |
-Macromolecule #8: Unknown fragment
| Macromolecule | Name: Unknown fragment / type: protein_or_peptide / ID: 8 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human adenovirus 26 Human adenovirus 26 |
| Molecular weight | Theoretical: 1.294587 KDa |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8.1 / Component - Concentration: 40.0 mM / Component - Formula: Tris / Component - Name: Tris / Details: 40 mM Tris pH 8.1 300 mM NaCl 10 mM CaCl2 |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 / Details: Blot for 3 seconds before plunging. |
| Details | Gradient purified virus |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 2 / Number real images: 2000 / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 150 / Target criteria: Correlation coefficient |
| Output model |  PDB-7tau: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)