[English] 日本語
 Yorodumi
Yorodumi- PDB-2lgi: Atomic Resolution Protein Structures using NMR Chemical Shift Tensors -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2lgi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Atomic Resolution Protein Structures using NMR Chemical Shift Tensors | ||||||
 Components Components | Immunoglobulin G-binding protein G | ||||||
 Keywords Keywords |  PROTEIN BINDING / GB1 / IMMUNOGLOBULIN BINDING DOMAIN / TEDOR / IGG-BINDING PROTEIN / PEPTIDOGLYCAN-ANCHOR / PROTEIN BINDING / GB1 / IMMUNOGLOBULIN BINDING DOMAIN / TEDOR / IGG-BINDING PROTEIN / PEPTIDOGLYCAN-ANCHOR /  SECRETED / SECRETED /  THERMOSTABLE THERMOSTABLE | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Streptococcus sp. group G (bacteria) Streptococcus sp. group G (bacteria) | ||||||
| Method | SOLID-STATE NMR /  simulated annealing simulated annealing | ||||||
| Model details | closest to the average, model 10 | ||||||
 Authors Authors | Wylie, B.J. / Sperling, L.J. / Nieuwkoop, A.J. / Franks, W.T. / Oldfield, E. / Rienstra, C.M. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2011 Journal: Proc.Natl.Acad.Sci.USA / Year: 2011Title: Ultrahigh resolution protein structures using NMR chemical shift tensors. Authors: Wylie, B.J. / Sperling, L.J. / Nieuwkoop, A.J. / Franks, W.T. / Oldfield, E. / Rienstra, C.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
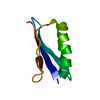
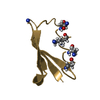
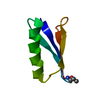
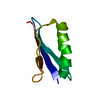
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2lgi.cif.gz 2lgi.cif.gz | 167.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2lgi.ent.gz pdb2lgi.ent.gz | 145.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2lgi.json.gz 2lgi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lg/2lgi https://data.pdbj.org/pub/pdb/validation_reports/lg/2lgi ftp://data.pdbj.org/pub/pdb/validation_reports/lg/2lgi ftp://data.pdbj.org/pub/pdb/validation_reports/lg/2lgi | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data | |
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Antibody | Mass: 6228.809 Da / Num. of mol.: 1 / Fragment: 2-1 repeat region residues 229-282 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Streptococcus sp. group G (bacteria) / Gene: spg / Production host: Streptococcus sp. group G (bacteria) / Gene: spg / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21 / References: UniProt: P06654 Escherichia coli (E. coli) / Strain (production host): BL21 / References: UniProt: P06654 |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLID-STATE NMR | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Contents: 20 mg U-2-13C-glycerol; U-100% 15N GB1 / Solvent system: solid |
|---|---|
| Sample | Conc.: 20 mg/mL / Component: GB1-1 / Isotopic labeling: U-2-13C-glycerol; U-100% 15N |
| Sample conditions | Ionic strength: 50 / pH: 5.5 / Pressure: ambient / Temperature: 273 K |
-NMR measurement
| NMR spectrometer | Type: Varian Infinity Plus / Manufacturer: Varian / Model : Infinity Plus / Field strength: 500 MHz : Infinity Plus / Field strength: 500 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method:  simulated annealing / Software ordinal: 1 simulated annealing / Software ordinal: 1 | ||||||||||||||||||||||||||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 200 / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller


















 PDBj
PDBj

