+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDEZ8 |
|---|---|
 Sample Sample | Interleukin-18 receptor accessory protein, IL-18Rβ-ECD
|
| Function / homology |  Function and homology information Function and homology informationinterleukin-18 receptor activity / interleukin-18 receptor complex / Interleukin-18 signaling / positive regulation of natural killer cell mediated cytotoxicity / interleukin-18-mediated signaling pathway / neutrophil activation / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NAD+ nucleosidase activity, cyclic ADP-ribose generating / coreceptor activity / : ...interleukin-18 receptor activity / interleukin-18 receptor complex / Interleukin-18 signaling / positive regulation of natural killer cell mediated cytotoxicity / interleukin-18-mediated signaling pathway / neutrophil activation / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NAD+ nucleosidase activity, cyclic ADP-ribose generating / coreceptor activity / : / cellular response to hydrogen peroxide / adaptive immune response / cell population proliferation / immune response / inflammatory response / cell surface / plasma membrane Similarity search - Function |
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
 Citation Citation |  Journal: Structure / Year: 2019 Journal: Structure / Year: 2019Title: Functional Relevance of Interleukin-1 Receptor Inter-domain Flexibility for Cytokine Binding and Signaling. Authors: Jiwan Ge / Soumya G Remesh / Michal Hammel / Si Pan / Andrew D Mahan / Shuying Wang / Xinquan Wang /    Abstract: The interleukin 1 (IL-1) receptor family, whose members contain three immunoglobulin-like domains (D1-D3) in the extracellular region, is responsible for transmitting pleiotropic signals of IL-1 ...The interleukin 1 (IL-1) receptor family, whose members contain three immunoglobulin-like domains (D1-D3) in the extracellular region, is responsible for transmitting pleiotropic signals of IL-1 cytokines. The inter-domain flexibility of IL-1 receptors and its functional roles have not been fully elucidated. In this study, we used small-angle X-ray scattering to show that ligand-binding primary receptors and co-receptors in the family all have inherent inter-domain flexibility due to the D2/D3 linker. Variants of the IL-1RAcP and IL-18Rβ co-receptors with mutated D2/D3 linkers cannot form a cytokine-receptor complex and mediate signaling. Our analysis further revealed that these mutated co-receptors exhibited a changed conformational ensemble, suggesting that loss of function is due to the alteration of receptor dynamics. Taken together, our results demonstrate that the D2/D3 linker is a critical functional determinant of IL-1 receptor and underscore the important roles of the inter-domain flexibility in cytokine/receptor binding and signaling. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDEZ8 SASDEZ8 |
|---|
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- External links
External links
| Related items in Molecule of the Month |
|---|
-Models
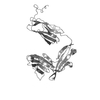
| Model #2663 |  Type: atomic / Chi-square value: 2.32931416757  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|---|
| Model #2664 |  Type: atomic / Chi-square value: 2.32931416757  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
- Sample
Sample
 Sample Sample | Name: Interleukin-18 receptor accessory protein, IL-18Rβ-ECD Specimen concentration: 10 mg/ml |
|---|---|
| Buffer | Name: 10mM HEPES, 150mM NaCl, 3% glycerol / pH: 7.2 |
| Entity #1396 | Type: protein / Description: Interleukin-18 receptor accessory protein / Formula weight: 41.363 / Num. of mol.: 1 / Source: Homo sapiens / References: UniProt: O95256 Sequence: FNISGCSTKK LLWTYSTRSE EEFVLFCDLP EPQKSHFCHR NRLSPKQVPE HLPFMGSNDL SDVQWYQQPS NGDPLEDIRK SYPHIIQDKC TLHFLTPGVN NSGSYICRPK MIKSPYDVAC CVKMILEVKP QTNASCEYSA SHKQDLLLGS TGSISCPSLS CQSDAQSPAV ...Sequence: FNISGCSTKK LLWTYSTRSE EEFVLFCDLP EPQKSHFCHR NRLSPKQVPE HLPFMGSNDL SDVQWYQQPS NGDPLEDIRK SYPHIIQDKC TLHFLTPGVN NSGSYICRPK MIKSPYDVAC CVKMILEVKP QTNASCEYSA SHKQDLLLGS TGSISCPSLS CQSDAQSPAV TWYKNGKLLS VERSNRIVVD EVYDYHQGTY VCDYTQSDTV SSWTVRAVVQ VRTIVGDTKL KPDILDPVED TLEVELGKPL TISCKARFGF ERVFNPVIKW YIKDSDLEWE VSVPEAKSIK STLKDEIIER NIILEKVTQR DLRRKFVCFV QNSIGNTTQS VQLKEKRAAA LHHILDAQKM VWNHRHHHHH H |
-Experimental information
| Beam | Instrument name: Advanced Light Source (ALS) 12.3.1 (SIBYLS) City: Berkeley, CA / 国: USA  / Type of source: X-ray synchrotron / Wavelength: 0.103 Å / Dist. spec. to detc.: 1.5 mm / Type of source: X-ray synchrotron / Wavelength: 0.103 Å / Dist. spec. to detc.: 1.5 mm | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus3 X 2M / Pixsize x: 172 mm | ||||||||||||||||||||||||||||||
| Scan | Measurement date: Jul 24, 2017 / Cell temperature: 20 °C / Exposure time: 3 sec. / Number of frames: 600 / Unit: 1/A /
| ||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||||||||||||||
| Result | Comments: SEC-SAXS was performed at 20°C using the following parameters: Column: Schodex kw-803 ; Flow rate: 0.5 mL/min; Total acquisition time: 30min; Sample injection concentration: 10 mg/mL; Injection volume: 50 μL.
|
 Movie
Movie Controller
Controller