[English] 日本語
 Yorodumi
Yorodumi- PDB-8k2d: Cryo-EM structure of the yeast 80S ribosome with tigecycline, eEF... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8k2d | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the yeast 80S ribosome with tigecycline, eEF2, Stm1 and eIF5A | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RIBOSOME / 80S ribosome / tigecycline / antibiotic | ||||||
| Function / homology |  Function and homology information Function and homology informationcytoplasmic translational elongation through polyproline stretches / positive regulation of cytoplasmic translational elongation through polyproline stretches / Hypusine synthesis from eIF5A-lysine / CAT tailing / translational frameshifting / cytoplasmic translational elongation / positive regulation of translational termination / Peptide chain elongation / Synthesis of diphthamide-EEF2 / triplex DNA binding ...cytoplasmic translational elongation through polyproline stretches / positive regulation of cytoplasmic translational elongation through polyproline stretches / Hypusine synthesis from eIF5A-lysine / CAT tailing / translational frameshifting / cytoplasmic translational elongation / positive regulation of translational termination / Peptide chain elongation / Synthesis of diphthamide-EEF2 / triplex DNA binding / Platelet degranulation / cytoplasmic translational termination / ribosome hibernation / translation elongation factor binding / positive regulation of translational elongation / regulation of translational initiation in response to stress / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, LSU-rRNA,5S) / regulation of amino acid metabolic process / negative regulation of glucose mediated signaling pathway / translational readthrough / positive regulation of translational fidelity / : / RMTs methylate histone arginines / Protein methylation / mTORC1-mediated signalling / Protein hydroxylation / ribosome-associated ubiquitin-dependent protein catabolic process / pre-mRNA 5'-splice site binding / GDP-dissociation inhibitor activity / cytosolic large ribosomal subunit assembly / positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / nonfunctional rRNA decay / Formation of the ternary complex, and subsequently, the 43S complex / Translation initiation complex formation / response to cycloheximide / Ribosomal scanning and start codon recognition / cleavage in ITS2 between 5.8S rRNA and LSU-rRNA of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / preribosome, small subunit precursor / Major pathway of rRNA processing in the nucleolus and cytosol / mRNA destabilization / telomeric DNA binding / SRP-dependent cotranslational protein targeting to membrane / GTP hydrolysis and joining of the 60S ribosomal subunit / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / positive regulation of protein kinase activity / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / L13a-mediated translational silencing of Ceruloplasmin expression / negative regulation of translational frameshifting / TOR signaling / positive regulation of translational initiation / ribosomal large subunit export from nucleus / translational elongation / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / G-protein alpha-subunit binding / Ub-specific processing proteases / 90S preribosome / translation elongation factor activity / ribosomal subunit export from nucleus / translational termination / regulation of translational fidelity / protein-RNA complex assembly / maturation of LSU-rRNA / translation repressor activity / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / ribosomal small subunit export from nucleus / translation regulator activity / Neutrophil degranulation / translation initiation factor activity / DNA-(apurinic or apyrimidinic site) endonuclease activity / rescue of stalled cytosolic ribosome / telomere maintenance / cellular response to amino acid starvation / protein kinase C binding / ribosome assembly / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / macroautophagy / maturation of SSU-rRNA / translational initiation / small-subunit processome / maintenance of translational fidelity / modification-dependent protein catabolic process / protein tag activity / cytoplasmic stress granule / rRNA processing / protein-folding chaperone binding / ribosome biogenesis / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | Buschauer, R. / Beckmann, R. / Cheng, J. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for differential inhibition of eukaryotic ribosomes by tigecycline. Authors: Xiang Li / Mengjiao Wang / Timo Denk / Robert Buschauer / Yi Li / Roland Beckmann / Jingdong Cheng /   Abstract: Tigecycline is widely used for treating complicated bacterial infections for which there are no effective drugs. It inhibits bacterial protein translation by blocking the ribosomal A-site. However, ...Tigecycline is widely used for treating complicated bacterial infections for which there are no effective drugs. It inhibits bacterial protein translation by blocking the ribosomal A-site. However, even though it is also cytotoxic for human cells, the molecular mechanism of its inhibition remains unclear. Here, we present cryo-EM structures of tigecycline-bound human mitochondrial 55S, 39S, cytoplasmic 80S and yeast cytoplasmic 80S ribosomes. We find that at clinically relevant concentrations, tigecycline effectively targets human 55S mitoribosomes, potentially, by hindering A-site tRNA accommodation and by blocking the peptidyl transfer center. In contrast, tigecycline does not bind to human 80S ribosomes under physiological concentrations. However, at high tigecycline concentrations, in addition to blocking the A-site, both human and yeast 80S ribosomes bind tigecycline at another conserved binding site restricting the movement of the L1 stalk. In conclusion, the observed distinct binding properties of tigecycline may guide new pathways for drug design and therapy. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8k2d.cif.gz 8k2d.cif.gz | 4.6 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8k2d.ent.gz pdb8k2d.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8k2d.json.gz 8k2d.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k2/8k2d https://data.pdbj.org/pub/pdb/validation_reports/k2/8k2d ftp://data.pdbj.org/pub/pdb/validation_reports/k2/8k2d ftp://data.pdbj.org/pub/pdb/validation_reports/k2/8k2d | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36839MC  8k2aC  8k2bC  8k2cC  8k82C  8xsxC  8xsyC  8xszC  8xt0C  8xt1C  8xt2C  8xt3C  8yooC  8yopC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-RNA chain , 4 types, 4 molecules C2C1C4C3
| #1: RNA chain | Mass: 579761.938 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: RNA chain | Mass: 1097493.875 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #3: RNA chain | Mass: 38951.105 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #4: RNA chain | Mass: 50682.922 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Small ribosomal subunit protein ... , 18 types, 18 molecules SASDSFSKSLSMSPSQSRSSSTSUSVSZSaScSdSg
| #5: Protein | Mass: 28051.330 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #8: Protein | Mass: 26542.789 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #10: Protein | Mass: 25072.600 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #15: Protein | Mass: 12757.445 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #16: Protein | Mass: 17785.934 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #17: Protein | Mass: 15488.631 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #20: Protein | Mass: 16031.907 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #21: Protein | Mass: 15877.490 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #22: Protein | Mass: 15820.413 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #23: Protein | Mass: 17071.641 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #24: Protein | Mass: 15942.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #25: Protein | Mass: 13929.044 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #26: Protein | Mass: 9758.829 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #30: Protein | Mass: 12067.272 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #31: Protein | Mass: 13480.886 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #33: Protein | Mass: 7605.847 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #34: Protein | Mass: 6675.723 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #37: Protein | Mass: 34841.219 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-40S ribosomal protein ... , 14 types, 14 molecules SBSCSESGSHSISJSNSOSWSXSYSbSe
| #6: Protein | Mass: 28798.467 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #7: Protein | Mass: 27490.826 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #9: Protein | Mass: 29469.330 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #11: Protein | Mass: 27054.486 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #12: Protein | Mass: 21658.209 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #13: Protein | Mass: 22537.803 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #14: Protein | Mass: 22487.893 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #18: Protein | Mass: 17059.945 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #19: Protein | Mass: 14562.655 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #27: Protein | Mass: 14650.062 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #28: Protein | Mass: 16073.896 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #29: Protein | Mass: 15362.848 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #32: Protein | Mass: 8893.391 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #35: Protein | Mass: 7137.541 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Ubiquitin-ribosomal protein ... , 2 types, 2 molecules SfLm
| #36: Protein | Mass: 17254.227 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #76: Protein | Mass: 14583.077 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
+Large ribosomal subunit protein ... , 43 types, 43 molecules LALBLCLDLELFLGLHLILJLKLLLMLNLOLPLQLRLSLTLULVLWLXLYLZLaLbLcLd...
-Protein , 3 types, 3 molecules CECDCS
| #80: Protein | Mass: 17222.406 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #83: Protein | Mass: 93407.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P32324, Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement |
| #84: Protein | Mass: 30048.010 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Non-polymers , 4 types, 20 molecules 
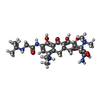





| #85: Chemical | ChemComp-ZN / #86: Chemical | ChemComp-T1C / #87: Chemical | ChemComp-MG / #88: Chemical | ChemComp-GDP / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: yeast 80S ribosome / Type: RIBOSOME / Entity ID: #1-#82, #84 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 3500 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 44 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| CTF correction | Details: Relion / Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 105095 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



















 PDBj
PDBj











































