+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7mfv | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
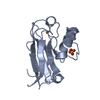
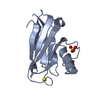
| Title | Crystal structure of synthetic nanobody (Sb16) | ||||||||||||
 Components Components | Synthetic Nanobody #16 (Sb16) | ||||||||||||
 Keywords Keywords | IMMUNE SYSTEM / SARS-CoV-2 / Spike Protein / RBD / Antibody / Nanobody / Sybody / VIRAL PROTEIN / epitope / neutralization | ||||||||||||
| Function / homology | Immunoglobulins / Immunoglobulin-like / Sandwich / Mainly Beta Function and homology information Function and homology information | ||||||||||||
| Biological species | synthetic construct (others) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||||||||
 Authors Authors | Jiang, J. / Ahmad, J. / Natarajan, K. / Boyd, L.F. / Margulies, D.H. | ||||||||||||
| Funding support |  United States, 1items United States, 1items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2021 Journal: J Biol Chem / Year: 2021Title: Structures of synthetic nanobody-SARS-CoV-2 receptor-binding domain complexes reveal distinct sites of interaction. Authors: Javeed Ahmad / Jiansheng Jiang / Lisa F Boyd / Allison Zeher / Rick Huang / Di Xia / Kannan Natarajan / David H Margulies /  Abstract: Combating the worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the emergence of new variants demands understanding of the structural basis of the interaction of ...Combating the worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the emergence of new variants demands understanding of the structural basis of the interaction of antibodies with the SARS-CoV-2 receptor-binding domain (RBD). Here, we report five X-ray crystal structures of sybodies (synthetic nanobodies) including those of binary and ternary complexes of Sb16-RBD, Sb45-RBD, Sb14-RBD-Sb68, and Sb45-RBD-Sb68, as well as unliganded Sb16. These structures reveal that Sb14, Sb16, and Sb45 bind the RBD at the angiotensin-converting enzyme 2 interface and that the Sb16 interaction is accompanied by a large conformational adjustment of complementarity-determining region 2. In contrast, Sb68 interacts at the periphery of the SARS-CoV-2 RBD-angiotensin-converting enzyme 2 interface. We also determined cryo-EM structures of Sb45 bound to the SARS-CoV-2 spike protein. Superposition of the X-ray structures of sybodies onto the trimeric spike protein cryo-EM map indicates that some sybodies may bind in both "up" and "down" configurations, but others may not. Differences in sybody recognition of several recently identified RBD variants are explained by these structures. #1: Journal: Biorxiv / Year: 2021 Title: Synthetic nanobody-SARS-CoV-2 receptor-binding domain structures identify distinct epitopes. Authors: Ahmad, J. / Jiang, J. / Boyd, L.F. / Natarajan, K. / Margulies, D.H. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7mfv.cif.gz 7mfv.cif.gz | 71.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7mfv.ent.gz pdb7mfv.ent.gz | 45.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7mfv.json.gz 7mfv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mf/7mfv https://data.pdbj.org/pub/pdb/validation_reports/mf/7mfv ftp://data.pdbj.org/pub/pdb/validation_reports/mf/7mfv ftp://data.pdbj.org/pub/pdb/validation_reports/mf/7mfv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7kgjC  7kgkC  7klwC  7mfuC  7n0gC  7n0hC  7kgl C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Antibody | Mass: 12873.515 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Plasmid: pET21b / Production host:  |
|---|---|
| #2: Chemical | ChemComp-EDO / |
| #3: Water | ChemComp-HOH / |
| Has ligand of interest | N |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.85 Å3/Da / Density % sol: 56.9 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7 / Details: 20% PEG 4000, 0.1M MES pH 6.0, 0.2M LiSO4 / PH range: 5.1 - 8.0 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Feb 5, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→40 Å / Num. obs: 12360 / % possible obs: 94.5 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 12.4 % / Biso Wilson estimate: 31.8 Å2 / CC1/2: 0.999 / CC star: 1 / Rmerge(I) obs: 0.0739 / Rpim(I) all: 0.0223 / Rrim(I) all: 0.0774 / Net I/σ(I): 15.19 |
| Reflection shell | Resolution: 1.9→1.97 Å / Redundancy: 12.6 % / Rmerge(I) obs: 1.8 / Mean I/σ(I) obs: 0.69 / Num. unique obs: 1025 / CC1/2: 0.526 / CC star: 0.83 / % possible all: 85 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7KGL  7kgl Resolution: 1.9→39.87 Å / SU ML: 0.2675 / Cross valid method: FREE R-VALUE / σ(F): 2 / Phase error: 31.1956 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 44.94 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→39.87 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION / Auth asym-ID: B / Label asym-ID: A
|
 Movie
Movie Controller
Controller













 PDBj
PDBj





