[English] 日本語
 Yorodumi
Yorodumi- PDB-7klr: Solution structure of the PHD1 domain of histone demethylase KDM5... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7klr | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
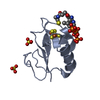
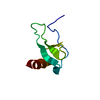
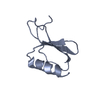
| Title | Solution structure of the PHD1 domain of histone demethylase KDM5A in complex with a histone H3(1-10) peptide | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | GENE REGULATION / PHD / H3 / Epigenetics / KDM5A | |||||||||
| Function / homology |  Function and homology information Function and homology information[histone H3]-trimethyl-L-lysine4 demethylase / histone H3K4me/H3K4me2/H3K4me3 demethylase activity / facultative heterochromatin formation / : / histone demethylase activity / Chromatin modifying enzymes / enzyme inhibitor activity / telomere organization / Interleukin-7 signaling / epigenetic regulation of gene expression ...[histone H3]-trimethyl-L-lysine4 demethylase / histone H3K4me/H3K4me2/H3K4me3 demethylase activity / facultative heterochromatin formation / : / histone demethylase activity / Chromatin modifying enzymes / enzyme inhibitor activity / telomere organization / Interleukin-7 signaling / epigenetic regulation of gene expression / RNA Polymerase I Promoter Opening / Assembly of the ORC complex at the origin of replication / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Negative Regulation of CDH1 Gene Transcription / circadian regulation of gene expression / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / protein-DNA complex / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / chromatin DNA binding / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Transcriptional regulation of granulopoiesis / RMTs methylate histone arginines / HCMV Early Events / structural constituent of chromatin / nucleosome / nucleosome assembly / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Senescence-Associated Secretory Phenotype (SASP) / histone binding / Oxidative Stress Induced Senescence / Estrogen-dependent gene expression / transcription coactivator activity / transcription cis-regulatory region binding / cadherin binding / chromatin remodeling / Amyloid fiber formation / protein heterodimerization activity / regulation of DNA-templated transcription / positive regulation of DNA-templated transcription / chromatin / nucleolus / negative regulation of transcription by RNA polymerase II / protein-containing complex / DNA binding / extracellular exosome / extracellular region / zinc ion binding / nucleoplasm / membrane / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | SOLUTION NMR / simulated annealing | |||||||||
 Authors Authors | Longbotham, E.J. / Kelly, M.J.S. / Fujimori, D.G. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Acs Chem.Biol. / Year: 2021 Journal: Acs Chem.Biol. / Year: 2021Title: Recognition of Histone H3 Methylation States by the PHD1 Domain of Histone Demethylase KDM5A. Authors: Longbotham, J.E. / Kelly, M.J.S. / Fujimori, D.G. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7klr.cif.gz 7klr.cif.gz | 417.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7klr.ent.gz pdb7klr.ent.gz | 345.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7klr.json.gz 7klr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kl/7klr https://data.pdbj.org/pub/pdb/validation_reports/kl/7klr ftp://data.pdbj.org/pub/pdb/validation_reports/kl/7klr ftp://data.pdbj.org/pub/pdb/validation_reports/kl/7klr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7kloC C: citing same article ( |
|---|---|
| Similar structure data | |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 6605.556 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KDM5A, JARID1A, RBBP2, RBP2 / Production host: Homo sapiens (human) / Gene: KDM5A, JARID1A, RBBP2, RBP2 / Production host:  References: UniProt: P29375, [histone H3]-trimethyl-L-lysine4 demethylase | ||
|---|---|---|---|
| #2: Protein/peptide | Mass: 1150.332 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: H3C1, H3FA, HIST1H3A, H3C2, H3FL, HIST1H3B, H3C3, H3FC HIST1H3C, H3C4, H3FB, HIST1H3D, H3C6, H3FD, HIST1H3E, H3C7, H3FI, HIST1H3F, H3C8, H3FH, HIST1H3G, H3C10, H3FK, HIST1H3H, H3C11, H3FF, ...Gene: H3C1, H3FA, HIST1H3A, H3C2, H3FL, HIST1H3B, H3C3, H3FC HIST1H3C, H3C4, H3FB, HIST1H3D, H3C6, H3FD, HIST1H3E, H3C7, H3FI, HIST1H3F, H3C8, H3FH, HIST1H3G, H3C10, H3FK, HIST1H3H, H3C11, H3FF, HIST1H3I, H3C12, H3FJ, HIST1H3J Production host: synthetic construct (others) / References: UniProt: P68431 | ||
| #3: Chemical | | Has ligand of interest | N | |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj










 HSQC
HSQC