+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7du0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of an type I-F anti-crispr protein | ||||||
 Components Components | AcrIF14 | ||||||
 Keywords Keywords | VIRAL PROTEIN / monomer / two-domain | ||||||
| Function / homology | Tail protein Function and homology information Function and homology information | ||||||
| Biological species |  Moraxella phage Mcat5 (virus) Moraxella phage Mcat5 (virus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.96 Å SAD / Resolution: 1.96 Å | ||||||
 Authors Authors | Teng, G. / Yue, F. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Insights into the dual functions of AcrIF14 during the inhibition of type I-F CRISPR-Cas surveillance complex. Authors: Xi Liu / Laixing Zhang / Yu Xiu / Teng Gao / Ling Huang / Yongchao Xie / Lingguang Yang / Wenhe Wang / Peiyi Wang / Yi Zhang / Maojun Yang / Yue Feng /  Abstract: CRISPR-Cas systems are bacterial adaptive immune systems, and phages counteract these systems using many approaches such as producing anti-CRISPR (Acr) proteins. Here, we report the structures of ...CRISPR-Cas systems are bacterial adaptive immune systems, and phages counteract these systems using many approaches such as producing anti-CRISPR (Acr) proteins. Here, we report the structures of both AcrIF14 and its complex with the crRNA-guided surveillance (Csy) complex. Our study demonstrates that apart from interacting with the Csy complex to block the hybridization of target DNA to the crRNA, AcrIF14 also endows the Csy complex with the ability to interact with non-sequence-specific dsDNA as AcrIF9 does. Further structural studies of the Csy-AcrIF14-dsDNA complex and biochemical studies uncover that the PAM recognition loop of the Cas8f subunit of the Csy complex and electropositive patches within the N-terminal domain of AcrIF14 are essential for the non-sequence-specific dsDNA binding to the Csy-AcrIF14 complex, which is different from the mechanism of AcrIF9. Our findings highlight the prevalence of Acr-induced non-specific DNA binding and shed light on future studies into the mechanisms of such Acr proteins. | ||||||
| History |
|
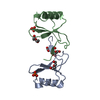
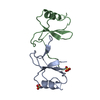
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7du0.cif.gz 7du0.cif.gz | 38.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7du0.ent.gz pdb7du0.ent.gz | 25.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7du0.json.gz 7du0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7du0_validation.pdf.gz 7du0_validation.pdf.gz | 426.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7du0_full_validation.pdf.gz 7du0_full_validation.pdf.gz | 429.7 KB | Display | |
| Data in XML |  7du0_validation.xml.gz 7du0_validation.xml.gz | 7.3 KB | Display | |
| Data in CIF |  7du0_validation.cif.gz 7du0_validation.cif.gz | 9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/du/7du0 https://data.pdbj.org/pub/pdb/validation_reports/du/7du0 ftp://data.pdbj.org/pub/pdb/validation_reports/du/7du0 ftp://data.pdbj.org/pub/pdb/validation_reports/du/7du0 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 14368.449 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Moraxella phage Mcat5 (virus) / Production host: Moraxella phage Mcat5 (virus) / Production host:  |
|---|---|
| #2: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.42 Å3/Da / Density % sol: 49.17 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion / Details: PEG 3350, sodium chloride |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U1 / Wavelength: 0.979 Å / Beamline: BL17U1 / Wavelength: 0.979 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Oct 12, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 1.96→50 Å / Num. obs: 17975 / % possible obs: 95.2 % / Redundancy: 5 % / CC1/2: 0.992 / Net I/σ(I): 24.5 |
| Reflection shell | Resolution: 1.96→2.05 Å / Num. unique obs: 1782 / CC1/2: 0.655 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.96→29.91 Å / SU ML: 0.16 / Cross valid method: THROUGHOUT / σ(F): 1.96 / Phase error: 33.18 / Stereochemistry target values: ML SAD / Resolution: 1.96→29.91 Å / SU ML: 0.16 / Cross valid method: THROUGHOUT / σ(F): 1.96 / Phase error: 33.18 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 75.2 Å2 / Biso mean: 44.0493 Å2 / Biso min: 21.24 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.96→29.91 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 6
|
 Movie
Movie Controller
Controller













 PDBj
PDBj
