+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7aki | ||||||
|---|---|---|---|---|---|---|---|
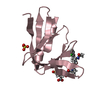
| Title | ENAH EVH1 in complex with Ac-[2-Cl-F]-[ProM-2]-[ProM-1]-NH2 | ||||||
 Components Components | Protein enabled homolog | ||||||
 Keywords Keywords | CELL INVASION / proline-rich motif / Ena/VASP inhibitor / actin / protein-protein interaction | ||||||
| Function / homology |  Function and homology information Function and homology informationactin polymerization-dependent cell motility / profilin binding / Signaling by ROBO receptors / actin polymerization or depolymerization / WW domain binding / Generation of second messenger molecules / filopodium / axon guidance / SH3 domain binding / cell junction ...actin polymerization-dependent cell motility / profilin binding / Signaling by ROBO receptors / actin polymerization or depolymerization / WW domain binding / Generation of second messenger molecules / filopodium / axon guidance / SH3 domain binding / cell junction / lamellipodium / actin binding / cytoskeleton / focal adhesion / synapse / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.36 Å MOLECULAR REPLACEMENT / Resolution: 1.36 Å | ||||||
 Authors Authors | Barone, M. / Roske, Y. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2020 Journal: Proc.Natl.Acad.Sci.USA / Year: 2020Title: Designed nanomolar small-molecule inhibitors of Ena/VASP EVH1 interaction impair invasion and extravasation of breast cancer cells. Authors: Barone, M. / Muller, M. / Chiha, S. / Ren, J. / Albat, D. / Soicke, A. / Dohmen, S. / Klein, M. / Bruns, J. / van Dinther, M. / Opitz, R. / Lindemann, P. / Beerbaum, M. / Motzny, K. / Roske, ...Authors: Barone, M. / Muller, M. / Chiha, S. / Ren, J. / Albat, D. / Soicke, A. / Dohmen, S. / Klein, M. / Bruns, J. / van Dinther, M. / Opitz, R. / Lindemann, P. / Beerbaum, M. / Motzny, K. / Roske, Y. / Schmieder, P. / Volkmer, R. / Nazare, M. / Heinemann, U. / Oschkinat, H. / Ten Dijke, P. / Schmalz, H.G. / Kuhne, R. #1:  Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2015 Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2015Title: A modular toolkit to inhibit proline-rich motif-mediated protein-protein interactions. Authors: Opitz, R. / Muller, M. / Reuter, C. / Barone, M. / Soicke, A. / Roske, Y. / Piotukh, K. / Huy, P. / Beerbaum, M. / Wiesner, B. / Beyermann, M. / Schmieder, P. / Freund, C. / Volkmer, R. / ...Authors: Opitz, R. / Muller, M. / Reuter, C. / Barone, M. / Soicke, A. / Roske, Y. / Piotukh, K. / Huy, P. / Beerbaum, M. / Wiesner, B. / Beyermann, M. / Schmieder, P. / Freund, C. / Volkmer, R. / Oschkinat, H. / Schmalz, H.G. / Kuhne, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7aki.cif.gz 7aki.cif.gz | 105.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7aki.ent.gz pdb7aki.ent.gz | 69 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7aki.json.gz 7aki.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7aki_validation.pdf.gz 7aki_validation.pdf.gz | 1.6 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7aki_full_validation.pdf.gz 7aki_full_validation.pdf.gz | 1.6 MB | Display | |
| Data in XML |  7aki_validation.xml.gz 7aki_validation.xml.gz | 9 KB | Display | |
| Data in CIF |  7aki_validation.cif.gz 7aki_validation.cif.gz | 11.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ak/7aki https://data.pdbj.org/pub/pdb/validation_reports/ak/7aki ftp://data.pdbj.org/pub/pdb/validation_reports/ak/7aki ftp://data.pdbj.org/pub/pdb/validation_reports/ak/7aki | HTTPS FTP |
-Related structure data
| Related structure data |  5n91SC  5n9cC  5n9pC  5najC  5nbfC  5nbxC  5nc2C  5nc7C  5ncfC  5ncgC  5ncpC  5nd0C  5nduC  5negC  6rcfC  6rcjC  6rd2C  6xvtC  6xxrC  7a5mC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 12628.273 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: M1A Q11A K21A are mutated for modelling as only the backbone was visible Source: (gene. exp.)  Homo sapiens (human) / Gene: ENAH, MENA Homo sapiens (human) / Gene: ENAH, MENAProduction host:  References: UniProt: Q8N8S7 | ||||
|---|---|---|---|---|---|
| #2: Chemical | ChemComp-NO3 / | ||||
| #3: Chemical | | #4: Water | ChemComp-HOH / | Has ligand of interest | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.84 Å3/Da / Density % sol: 33.3 % |
|---|---|
| Crystal grow | Temperature: 300.15 K / Method: vapor diffusion, sitting drop / pH: 7 / Details: 1.541M ammonium sulfate, 564mM potassium bromide / Temp details: incubator |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.2 / Wavelength: 0.9184 Å / Beamline: 14.2 / Wavelength: 0.9184 Å |
| Detector | Type: DECTRIS PILATUS 2M / Detector: PIXEL / Date: Dec 14, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9184 Å / Relative weight: 1 |
| Reflection | Resolution: 1.36→44.53 Å / Num. obs: 20643 / % possible obs: 100 % / Redundancy: 14.2 % / Biso Wilson estimate: 22.73 Å2 / CC1/2: 0.999 / Rrim(I) all: 0.131 / Net I/σ(I): 11.48 |
| Reflection shell | Resolution: 1.36→1.47 Å / Redundancy: 14.5 % / Num. unique obs: 4246 / CC1/2: 0.273 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5N91 Resolution: 1.36→44.53 Å / SU ML: 0.2564 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 31.9997 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.8 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.36→44.53 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj