+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6y0g | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human ribosome in classical-PRE state | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | RIBOSOME / Classical-PRE state / Cycloheximide | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtranslation at presynapse / exit from mitosis / optic nerve development / response to insecticide / eukaryotic 80S initiation complex / negative regulation of protein neddylation / regulation of translation involved in cellular response to UV / axial mesoderm development / negative regulation of formation of translation preinitiation complex / regulation of G1 to G0 transition ...translation at presynapse / exit from mitosis / optic nerve development / response to insecticide / eukaryotic 80S initiation complex / negative regulation of protein neddylation / regulation of translation involved in cellular response to UV / axial mesoderm development / negative regulation of formation of translation preinitiation complex / regulation of G1 to G0 transition / retinal ganglion cell axon guidance / oxidized pyrimidine DNA binding / response to TNF agonist / negative regulation of endoplasmic reticulum unfolded protein response / positive regulation of base-excision repair / ribosomal protein import into nucleus / protein-DNA complex disassembly / positive regulation of respiratory burst involved in inflammatory response / positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage / positive regulation of gastrulation / 90S preribosome assembly / protein tyrosine kinase inhibitor activity / positive regulation of endodeoxyribonuclease activity / nucleolus organization / IRE1-RACK1-PP2A complex / positive regulation of Golgi to plasma membrane protein transport / TNFR1-mediated ceramide production / alpha-beta T cell differentiation / negative regulation of DNA repair / negative regulation of RNA splicing / GAIT complex / positive regulation of DNA damage response, signal transduction by p53 class mediator / TORC2 complex binding / G1 to G0 transition / supercoiled DNA binding / NF-kappaB complex / neural crest cell differentiation / oxidized purine DNA binding / cysteine-type endopeptidase activator activity involved in apoptotic process / middle ear morphogenesis / positive regulation of ubiquitin-protein transferase activity / negative regulation of intrinsic apoptotic signaling pathway in response to hydrogen peroxide / negative regulation of bicellular tight junction assembly / regulation of establishment of cell polarity / ubiquitin-like protein conjugating enzyme binding / rRNA modification in the nucleus and cytosol / negative regulation of phagocytosis / erythrocyte homeostasis / Formation of the ternary complex, and subsequently, the 43S complex / cytoplasmic side of rough endoplasmic reticulum membrane / negative regulation of ubiquitin protein ligase activity / protein kinase A binding / laminin receptor activity / homeostatic process / ion channel inhibitor activity / Ribosomal scanning and start codon recognition / pigmentation / Translation initiation complex formation / positive regulation of mitochondrial depolarization / macrophage chemotaxis / lung morphogenesis / positive regulation of T cell receptor signaling pathway / fibroblast growth factor binding / negative regulation of Wnt signaling pathway / positive regulation of natural killer cell proliferation / male meiosis I / monocyte chemotaxis / TOR signaling / negative regulation of translational frameshifting / BH3 domain binding / positive regulation of activated T cell proliferation / Protein hydroxylation / SARS-CoV-1 modulates host translation machinery / iron-sulfur cluster binding / regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / regulation of cell division / cellular response to ethanol / mTORC1-mediated signalling / Peptide chain elongation / Selenocysteine synthesis / Formation of a pool of free 40S subunits / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / cellular response to actinomycin D / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / Eukaryotic Translation Termination / blastocyst development / positive regulation of GTPase activity / negative regulation of ubiquitin-dependent protein catabolic process / protein serine/threonine kinase inhibitor activity / SRP-dependent cotranslational protein targeting to membrane / Response of EIF2AK4 (GCN2) to amino acid deficiency / ubiquitin ligase inhibitor activity / Viral mRNA Translation / negative regulation of respiratory burst involved in inflammatory response / positive regulation of signal transduction by p53 class mediator / protein localization to nucleus / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Bhaskar, V. / Schenk, A.D. / Cavadini, S. / von Loeffelholz, O. / Natchiar, S.K. / Klaholz, B.P. / Chao, J.A. | ||||||||||||
| Funding support |  Switzerland, Switzerland,  France, 3items France, 3items
| ||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2020 Journal: Cell Rep / Year: 2020Title: Dynamics of uS19 C-Terminal Tail during the Translation Elongation Cycle in Human Ribosomes. Authors: Varun Bhaskar / Alexandra Graff-Meyer / Andreas D Schenk / Simone Cavadini / Ottilie von Loeffelholz / S Kundhavai Natchiar / Caroline G Artus-Revel / Hans-Rudolf Hotz / Gabriel Bretones / ...Authors: Varun Bhaskar / Alexandra Graff-Meyer / Andreas D Schenk / Simone Cavadini / Ottilie von Loeffelholz / S Kundhavai Natchiar / Caroline G Artus-Revel / Hans-Rudolf Hotz / Gabriel Bretones / Bruno P Klaholz / Jeffrey A Chao /    Abstract: Ribosomes undergo multiple conformational transitions during translation elongation. Here, we report the high-resolution cryoelectron microscopy (cryo-EM) structure of the human 80S ribosome in the ...Ribosomes undergo multiple conformational transitions during translation elongation. Here, we report the high-resolution cryoelectron microscopy (cryo-EM) structure of the human 80S ribosome in the post-decoding pre-translocation state (classical-PRE) at 3.3-Å resolution along with the rotated (hybrid-PRE) and the post-translocation states (POST). The classical-PRE state ribosome structure reveals a previously unobserved interaction between the C-terminal region of the conserved ribosomal protein uS19 and the A- and P-site tRNAs and the mRNA in the decoding site. In addition to changes in the inter-subunit bridges, analysis of different ribosomal conformations reveals the dynamic nature of this domain and suggests a role in tRNA accommodation and translocation during elongation. Furthermore, we show that disease-associated mutations in uS19 result in increased frameshifting. Together, this structure-function analysis provides mechanistic insights into the role of the uS19 C-terminal tail in the context of mammalian ribosomes. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6y0g.cif.gz 6y0g.cif.gz | 4.7 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6y0g.ent.gz pdb6y0g.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  6y0g.json.gz 6y0g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y0/6y0g https://data.pdbj.org/pub/pdb/validation_reports/y0/6y0g ftp://data.pdbj.org/pub/pdb/validation_reports/y0/6y0g ftp://data.pdbj.org/pub/pdb/validation_reports/y0/6y0g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10668MC  6y2lC  6y57C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-RNA chain , 6 types, 7 molecules A4B4D4L5L7L8S2
| #1: RNA chain | Mass: 8527.686 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 Homo sapiens (human) / Cell line: HEK293 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: RNA chain | Mass: 24485.539 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 Homo sapiens (human) / Cell line: HEK293#4: RNA chain | | Mass: 1640182.000 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK-293T and Expi-293F Homo sapiens (human) / Cell line: HEK-293T and Expi-293F#5: RNA chain | | Mass: 38691.914 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 / References: GenBank: 23898 Homo sapiens (human) / Cell line: HEK293 / References: GenBank: 23898#6: RNA chain | | Mass: 50143.648 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 Homo sapiens (human) / Cell line: HEK293#49: RNA chain | | Mass: 602752.875 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 / References: GenBank: 36162 Homo sapiens (human) / Cell line: HEK293 / References: GenBank: 36162 |
-Protein/peptide , 1 types, 1 molecules C4
| #3: Protein/peptide | Mass: 188.225 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 Homo sapiens (human) / Cell line: HEK293 |
|---|
+60S ribosomal protein ... , 41 types, 41 molecules LALBLCLDLELFLGLHLILJLLLMLNLOLPLQLRLSLTLULVLWLXLYLZLaLbLcLdLe...
-Protein , 3 types, 3 molecules LmSfSg
| #44: Protein | Mass: 14758.394 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P62987 Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P62987 |
|---|---|
| #81: Protein | Mass: 18004.041 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P62979 Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P62979 |
| #82: Protein | Mass: 35115.652 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P63244 Homo sapiens (human) / Cell line: HEK293 / References: UniProt: P63244 |
+40S ribosomal protein ... , 31 types, 31 molecules SASBSCSDSESFSGSHSISJSKSLSMSNSOSPSQSRSSSTSUSVSWSXSYSZSaSbScSdSe
-Non-polymers , 4 types, 92 molecules 
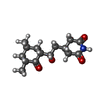





| #83: Chemical | ChemComp-MG / #84: Chemical | ChemComp-3HE / | #85: Chemical | ChemComp-ZN / #86: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human ribosomes in the classical-PRE state / Type: RIBOSOME / Entity ID: #1-#82 / Source: NATURAL | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 4.3 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.6 | ||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/2 | ||||||||||||||||||||||||||||||
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 80 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 40 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 332383 | ||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 21820 / Symmetry type: POINT | ||||||||||||||||||||||||||||
| Atomic model building | Protocol: OTHER / Space: REAL | ||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 91.75 Å2 | ||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
























 PDBj
PDBj

























































