+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 6o1v | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Complex of human cystic fibrosis transmembrane conductance regulator (CFTR) and GLPG1837 | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | HYDROLASE / ABC transporter / anion channel / cystic fibrosis / membrane protein / GLPG1837 | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報positive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / positive regulation of enamel mineralization / transepithelial water transport / RHO GTPases regulate CFTR trafficking / amelogenesis / intracellular pH elevation ...positive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / positive regulation of enamel mineralization / transepithelial water transport / RHO GTPases regulate CFTR trafficking / amelogenesis / intracellular pH elevation / chloride channel inhibitor activity / : / Golgi-associated vesicle membrane / multicellular organismal-level water homeostasis / cholesterol transport / bicarbonate transport / bicarbonate transmembrane transporter activity / vesicle docking involved in exocytosis / chloride channel regulator activity / membrane hyperpolarization / chloride transmembrane transporter activity / sperm capacitation / cholesterol biosynthetic process / RHOQ GTPase cycle / chloride channel activity / positive regulation of exocytosis / ATPase-coupled transmembrane transporter activity / chloride channel complex / positive regulation of insulin secretion involved in cellular response to glucose stimulus / ABC-type transporter activity / 14-3-3 protein binding / cellular response to forskolin / chloride transmembrane transport / response to endoplasmic reticulum stress / cellular response to cAMP / PDZ domain binding / establishment of localization in cell / clathrin-coated endocytic vesicle membrane / Defective CFTR causes cystic fibrosis / Late endosomal microautophagy / recycling endosome / ABC-family proteins mediated transport / transmembrane transport / recycling endosome membrane / Chaperone Mediated Autophagy / Aggrephagy / Cargo recognition for clathrin-mediated endocytosis / Clathrin-mediated endocytosis / protein-folding chaperone binding / early endosome membrane / early endosome / endosome membrane / Ub-specific processing proteases / apical plasma membrane / lysosomal membrane / endoplasmic reticulum membrane / enzyme binding / cell surface / protein-containing complex / ATP hydrolysis activity / ATP binding / nucleus / membrane / plasma membrane / cytoplasm / cytosol 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.2 Å | ||||||
 データ登録者 データ登録者 | Zhang, Z. / Liu, F. / Chen, J. / Levit, A. / Shoichet, B. | ||||||
| 資金援助 |  米国, 1件 米国, 1件
| ||||||
 引用 引用 |  ジャーナル: Science / 年: 2019 ジャーナル: Science / 年: 2019タイトル: Structural identification of a hotspot on CFTR for potentiation. 著者: Fangyu Liu / Zhe Zhang / Anat Levit / Jesper Levring / Kouki K Touhara / Brian K Shoichet / Jue Chen /  要旨: Cystic fibrosis is a fatal disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Two main categories of drugs are being developed: correctors that improve ...Cystic fibrosis is a fatal disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Two main categories of drugs are being developed: correctors that improve folding of CFTR and potentiators that recover the function of CFTR. Here, we report two cryo-electron microscopy structures of human CFTR in complex with potentiators: one with the U.S. Food and Drug Administration (FDA)-approved drug ivacaftor at 3.3-angstrom resolution and the other with an investigational drug, GLPG1837, at 3.2-angstrom resolution. These two drugs, although chemically dissimilar, bind to the same site within the transmembrane region. Mutagenesis suggests that in both cases, hydrogen bonds provided by the protein are important for drug recognition. The molecular details of how ivacaftor and GLPG1837 interact with CFTR may facilitate structure-based optimization of therapeutic compounds. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  6o1v.cif.gz 6o1v.cif.gz | 264.4 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb6o1v.ent.gz pdb6o1v.ent.gz | 202.6 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  6o1v.json.gz 6o1v.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  6o1v_validation.pdf.gz 6o1v_validation.pdf.gz | 1.1 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  6o1v_full_validation.pdf.gz 6o1v_full_validation.pdf.gz | 1.1 MB | 表示 | |
| XML形式データ |  6o1v_validation.xml.gz 6o1v_validation.xml.gz | 37.5 KB | 表示 | |
| CIF形式データ |  6o1v_validation.cif.gz 6o1v_validation.cif.gz | 56.4 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v https://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v ftp://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v ftp://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v | HTTPS FTP |
-関連構造データ
| 関連構造データ |  0606MC  0611C  6o2pC M: このデータのモデリングに利用したマップデータ C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| 電子顕微鏡画像生データ |  EMPIAR-10281 (タイトル: Cryo electron micrographs of human cystic fibrosis transmembrane conductance regulator (CFTR) in complex with GLPG EMPIAR-10281 (タイトル: Cryo electron micrographs of human cystic fibrosis transmembrane conductance regulator (CFTR) in complex with GLPGData size: 1.4 TB Data #1: Unaligned multi-frame micrographs of human CFTR in complex with GLPG1837 [micrographs - multiframe]) |
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
-タンパク質 / タンパク質・ペプチド , 2種, 2分子 AB
| #1: タンパク質 | 分子量: 169352.594 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: CFTR, ABCC7 / 発現宿主: Homo sapiens (ヒト) / 遺伝子: CFTR, ABCC7 / 発現宿主:  Homo sapiens (ヒト) / 参照: UniProt: P13569, EC: 3.6.3.49 Homo sapiens (ヒト) / 参照: UniProt: P13569, EC: 3.6.3.49 |
|---|---|
| #2: タンパク質・ペプチド | 分子量: 1464.797 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 発現宿主: Homo sapiens (ヒト) / 発現宿主:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-非ポリマー , 5種, 11分子 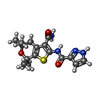








| #3: 化合物 | ChemComp-LJP / | ||||||
|---|---|---|---|---|---|---|---|
| #4: 化合物 | | #5: 化合物 | #6: 化合物 | ChemComp-POV / ( #7: 化合物 | ChemComp-CLR / | |
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: Complex of human cystic fibrosis transmembrane conductance regulator (CFTR) and GLPG1837 タイプ: COMPLEX / Entity ID: #1-#2 / 由来: RECOMBINANT |
|---|---|
| 分子量 | 値: 0.168 MDa / 実験値: NO |
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 由来(組換発現) | 生物種:  Homo sapiens (ヒト) / 細胞: HEK293S GnTI- / プラスミド: BacMam Homo sapiens (ヒト) / 細胞: HEK293S GnTI- / プラスミド: BacMam |
| 緩衝液 | pH: 7.5 |
| 試料 | 濃度: 5.5 mg/ml / 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES |
| 試料支持 | 詳細: unspecified |
| 急速凍結 | 装置: FEI VITROBOT MARK I / 凍結剤: ETHANE / 湿度: 100 % / 凍結前の試料温度: 298 K |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD |
| 撮影 | 電子線照射量: 1.51 e/Å2 フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) |
- 解析
解析
| EMソフトウェア |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF補正 | タイプ: NONE | |||||||||||||||||||||||||||
| 粒子像の選択 | 選択した粒子像数: 510483 | |||||||||||||||||||||||||||
| 対称性 | 点対称性: C1 (非対称) | |||||||||||||||||||||||||||
| 3次元再構成 | 解像度: 3.2 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 510483 / 対称性のタイプ: POINT | |||||||||||||||||||||||||||
| 原子モデル構築 | B value: 150 / プロトコル: AB INITIO MODEL / 空間: RECIPROCAL | |||||||||||||||||||||||||||
| 原子モデル構築 | PDB-ID: 6MSM Accession code: 6MSM / Source name: PDB / タイプ: experimental model |
 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj














