[English] 日本語
 Yorodumi
Yorodumi- EMDB-20945: Cryo-EM structure of wild-type bovine multidrug resistance protei... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20945 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of wild-type bovine multidrug resistance protein 1 (MRP1) under active turnover conditions | |||||||||
 Map data Map data | 3.23-Angstrom cryo-EM reconstruction of wild-type bovine MRP1 under active turnover conditions in the presence of ATP and leukotriene C4 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / multidrug resistance / outward facing / TRANSPORT PROTEIN / MRP1 | |||||||||
| Function / homology |  Function and homology information Function and homology informationSphingolipid de novo biosynthesis / Heme degradation / Synthesis of Leukotrienes (LT) and Eoxins (EX) / Transport of RCbl within the body / cyclic nucleotide transport / Paracetamol ADME / ABC-family proteins mediated transport / glutathione transmembrane transport / leukotriene transport / ABC-type glutathione S-conjugate transporter activity ...Sphingolipid de novo biosynthesis / Heme degradation / Synthesis of Leukotrienes (LT) and Eoxins (EX) / Transport of RCbl within the body / cyclic nucleotide transport / Paracetamol ADME / ABC-family proteins mediated transport / glutathione transmembrane transport / leukotriene transport / ABC-type glutathione S-conjugate transporter activity / Cytoprotection by HMOX1 / ABC-type glutathione-S-conjugate transporter / glutathione transmembrane transporter activity / ABC-type xenobiotic transporter / ABC-type xenobiotic transporter activity / lipid transport / xenobiotic transmembrane transporter activity / xenobiotic transport / ABC-type transporter activity / positive regulation of inflammatory response / basolateral plasma membrane / response to xenobiotic stimulus / ATP hydrolysis activity / ATP binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
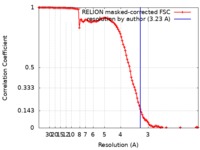
| Method | single particle reconstruction / cryo EM / Resolution: 3.23 Å | |||||||||
 Authors Authors | Johnson ZL / Chen J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. Authors: Ling Wang / Zachary Lee Johnson / Michael R Wasserman / Jesper Levring / Jue Chen / Shixin Liu /  Abstract: ATP-binding cassette (ABC) transporters are molecular pumps ubiquitous across all kingdoms of life. While their structures have been widely reported, the kinetics governing their transport cycles ...ATP-binding cassette (ABC) transporters are molecular pumps ubiquitous across all kingdoms of life. While their structures have been widely reported, the kinetics governing their transport cycles remain largely unexplored. Multidrug resistance protein 1 (MRP1) is an ABC exporter that extrudes a variety of chemotherapeutic agents and native substrates. Previously, the structures of MRP1 were determined in an inward-facing (IF) or outward-facing (OF) conformation. Here, we used single-molecule fluorescence spectroscopy to track the conformational changes of bovine MRP1 (bMRP1) in real time. We also determined the structure of bMRP1 under active turnover conditions. Our results show that substrate stimulates ATP hydrolysis by accelerating the IF-to-OF transition. The rate-limiting step of the transport cycle is the dissociation of the nucleotide-binding-domain dimer, while ATP hydrolysis per se does not reset MRP1 to the resting state. The combination of structural and kinetic data illustrates how different conformations of MRP1 are temporally linked and how substrate and ATP alter protein dynamics to achieve active transport. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20945.map.gz emd_20945.map.gz | 117.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20945-v30.xml emd-20945-v30.xml emd-20945.xml emd-20945.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20945_fsc.xml emd_20945_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_20945.png emd_20945.png | 126.2 KB | ||
| Filedesc metadata |  emd-20945.cif.gz emd-20945.cif.gz | 7.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20945 http://ftp.pdbj.org/pub/emdb/structures/EMD-20945 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20945 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20945 | HTTPS FTP |
-Related structure data
| Related structure data |  6uy0MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20945.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20945.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3.23-Angstrom cryo-EM reconstruction of wild-type bovine MRP1 under active turnover conditions in the presence of ATP and leukotriene C4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : bovine multidrug resistance protein 1 (MRP1)
| Entire | Name: bovine multidrug resistance protein 1 (MRP1) |
|---|---|
| Components |
|
-Supramolecule #1: bovine multidrug resistance protein 1 (MRP1)
| Supramolecule | Name: bovine multidrug resistance protein 1 (MRP1) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 170 KDa |
-Macromolecule #1: Multidrug resistance-associated protein 1
| Macromolecule | Name: Multidrug resistance-associated protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: ABC-type xenobiotic transporter |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 172.862562 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MALRDFCSVD GSDLFWEWNV TWNTSNPDFT KCFQNTVLVW VPCSYLWVCF PFYFLYLSHH DRGYIQMTHL NKAKTALGFL LWIVCWADL FYSFWERSMG KLLAPVFLVS PTLLGITMLL ATFLIQIERR RGVQSSGIML TFWLIALLCA LAILRSKIMT A LKEDARVD ...String: MALRDFCSVD GSDLFWEWNV TWNTSNPDFT KCFQNTVLVW VPCSYLWVCF PFYFLYLSHH DRGYIQMTHL NKAKTALGFL LWIVCWADL FYSFWERSMG KLLAPVFLVS PTLLGITMLL ATFLIQIERR RGVQSSGIML TFWLIALLCA LAILRSKIMT A LKEDARVD VFRDVTFYIY FSLVLIQLVL SCFSDRSPLF SETINDPNPC PESSASFLSR ITFWWITGMM VQGYRQPLES TD LWSLNKE DTSEQVVPVL VKNWKKECAK SRKQPVKIVY SSKDPAKPKG SSKVDVNEEA EALIVKCPQK ERDPSLFKVL YKT FGPYFL MSFLFKAVHD LMMFAGPEIL KLLINFVNDK KAPEWQGYFY TALLFISACL QTLVLHQYFH ICFVSGMRIK TAVI GAVYR KALVITNAAR KSSTVGEIVN LMSVDAQRFM DLATYINMIW SAPLQVILAL YLLWLNLGPS VLAGVAVMVL MVPLN AVMA MKTKTYQVAH MKSKDNRIKL MNEILNGIKV LKLYAWELAF KDKVLAIRQE ELKVLKKSAY LAAVGTFTWV CTPFLV ALS TFAVYVTVDE NNILDAQKAF VSLALFNILR FPLNILPMVI SSIVQASVSL KRLRVFLSHE DLDPDSIQRR PIKDAGA TN SITVKNATFT WARNDPPTLH GITFSVPEGS LVAVVGQVGC GKSSLLSALL AEMDKVEGHV TVKGSVAYVP QQAWIQNI S LRENILFGRQ LQERYYKAVV EACALLPDLE ILPSGDRTEI GEKGVNLSGG QKQRVSLARA VYCDSDVYLL DDPLSAVDA HVGKHIFENV IGPKGLLKNK TRLLVTHAIS YLPQMDVIIV MSGGKISEMG SYQELLARDG AFAEFLRTYA SAEQEQGQPE DGLAGVGGP GKEVKQMENG MLVTDTAGKQ MQRQLSSSSS YSRDVSQHHT STAELRKPGP TEETWKLVEA DKAQTGQVKL S VYWDYMKA IGLFISFLSI FLFLCNHVAS LVSNYWLSLW TDDPIVNGTQ EHTQVRLSVY GALGISQGIT VFGYSMAVSI GG IFASRRL HLDLLHNVLR SPISFFERTP SGNLVNRFSK ELDTVDSMIP QVIKMFMGSL FNVIGACIII LLATPMAAVI IPP LGLIYF FVQRFYVASS RQLKRLESVS RSPVYSHFNE TLLGVSVIRA FEEQERFIRQ SDLKVDENQK AYYPSIVANR WLAV RLECV GNCIVLFASL FAVISRHSLS AGLVGLSVSY SLQVTTYLNW LVRMSSEMET NIVAVERLKE YSETEKEAPW QIQDM APPK DWPQVGRVEF RDYGLRYRED LDLVLKHINV TIDGGEKVGI VGRTGAGKSS LTLGLFRIKE SAEGEIIIDD INIAKI GLH DLRFKITIIP QDPVLFSGSL RMNLDPFSQY SDEEVWTSLE LAHLKGFVSA LPDKLNHECA EGGENLSVGQ RQLVCLA RA LLRKTKILVL DEATAAVDLE TDDLIQSTIR TQFDDCTVLT IAHRLNTIMD YTRVIVLDKG EIQEWGSPSD LLQQRGLF Y SMAKDSGLVS NSLEVLFQ UniProtKB: Multidrug resistance-associated protein 1 |
-Macromolecule #2: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 1 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 5 / Number of copies: 3 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.3 mg/mL | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 12 sec. | ||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 80.0 K / Max: 100.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Digitization - Frames/image: 1-50 / Number grids imaged: 1 / Number real images: 3993 / Average exposure time: 10.0 sec. / Average electron dose: 75.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)