[English] 日本語
 Yorodumi
Yorodumi- PDB-6o1v: Complex of human cystic fibrosis transmembrane conductance regula... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6o1v | ||||||
|---|---|---|---|---|---|---|---|
| Title | Complex of human cystic fibrosis transmembrane conductance regulator (CFTR) and GLPG1837 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE / ABC transporter / anion channel / cystic fibrosis / membrane protein / GLPG1837 | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / positive regulation of enamel mineralization / transepithelial water transport / RHO GTPases regulate CFTR trafficking / amelogenesis / intracellular pH elevation ...positive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / positive regulation of enamel mineralization / transepithelial water transport / RHO GTPases regulate CFTR trafficking / amelogenesis / intracellular pH elevation / chloride channel inhibitor activity / : / multicellular organismal-level water homeostasis / Golgi-associated vesicle membrane / cholesterol transport / bicarbonate transport / bicarbonate transmembrane transporter activity / chloride channel regulator activity / membrane hyperpolarization / vesicle docking involved in exocytosis / chloride transmembrane transporter activity / cholesterol biosynthetic process / sperm capacitation / chloride channel activity / RHOQ GTPase cycle / positive regulation of exocytosis / ATPase-coupled transmembrane transporter activity / ABC-type transporter activity / chloride channel complex / positive regulation of insulin secretion involved in cellular response to glucose stimulus / 14-3-3 protein binding / cellular response to forskolin / chloride transmembrane transport / cellular response to cAMP / response to endoplasmic reticulum stress / PDZ domain binding / establishment of localization in cell / clathrin-coated endocytic vesicle membrane / Defective CFTR causes cystic fibrosis / Late endosomal microautophagy / recycling endosome / ABC-family proteins mediated transport / transmembrane transport / recycling endosome membrane / Chaperone Mediated Autophagy / Aggrephagy / Cargo recognition for clathrin-mediated endocytosis / protein-folding chaperone binding / Clathrin-mediated endocytosis / early endosome membrane / early endosome / endosome membrane / Ub-specific processing proteases / apical plasma membrane / lysosomal membrane / endoplasmic reticulum membrane / enzyme binding / cell surface / ATP hydrolysis activity / protein-containing complex / ATP binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | Zhang, Z. / Liu, F. / Chen, J. / Levit, A. / Shoichet, B. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Structural identification of a hotspot on CFTR for potentiation. Authors: Fangyu Liu / Zhe Zhang / Anat Levit / Jesper Levring / Kouki K Touhara / Brian K Shoichet / Jue Chen /  Abstract: Cystic fibrosis is a fatal disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Two main categories of drugs are being developed: correctors that improve ...Cystic fibrosis is a fatal disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Two main categories of drugs are being developed: correctors that improve folding of CFTR and potentiators that recover the function of CFTR. Here, we report two cryo-electron microscopy structures of human CFTR in complex with potentiators: one with the U.S. Food and Drug Administration (FDA)-approved drug ivacaftor at 3.3-angstrom resolution and the other with an investigational drug, GLPG1837, at 3.2-angstrom resolution. These two drugs, although chemically dissimilar, bind to the same site within the transmembrane region. Mutagenesis suggests that in both cases, hydrogen bonds provided by the protein are important for drug recognition. The molecular details of how ivacaftor and GLPG1837 interact with CFTR may facilitate structure-based optimization of therapeutic compounds. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6o1v.cif.gz 6o1v.cif.gz | 264.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6o1v.ent.gz pdb6o1v.ent.gz | 202.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6o1v.json.gz 6o1v.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v https://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v ftp://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v ftp://data.pdbj.org/pub/pdb/validation_reports/o1/6o1v | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  0606MC  0611C  6o2pC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10281 (Title: Cryo electron micrographs of human cystic fibrosis transmembrane conductance regulator (CFTR) in complex with GLPG EMPIAR-10281 (Title: Cryo electron micrographs of human cystic fibrosis transmembrane conductance regulator (CFTR) in complex with GLPGData size: 1.4 TB Data #1: Unaligned multi-frame micrographs of human CFTR in complex with GLPG1837 [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 2 molecules AB
| #1: Protein | Mass: 169352.594 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CFTR, ABCC7 / Production host: Homo sapiens (human) / Gene: CFTR, ABCC7 / Production host:  Homo sapiens (human) / References: UniProt: P13569, EC: 3.6.3.49 Homo sapiens (human) / References: UniProt: P13569, EC: 3.6.3.49 |
|---|---|
| #2: Protein/peptide | Mass: 1464.797 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
-Non-polymers , 5 types, 11 molecules 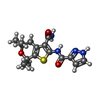








| #3: Chemical | ChemComp-LJP / | ||||||
|---|---|---|---|---|---|---|---|
| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-POV / ( #7: Chemical | ChemComp-CLR / | |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Complex of human cystic fibrosis transmembrane conductance regulator (CFTR) and GLPG1837 Type: COMPLEX / Entity ID: #1-#2 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.168 MDa / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) / Cell: HEK293S GnTI- / Plasmid: BacMam Homo sapiens (human) / Cell: HEK293S GnTI- / Plasmid: BacMam |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 5.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: unspecified |
| Vitrification | Instrument: FEI VITROBOT MARK I / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 298 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 1.51 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | |||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 510483 | |||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | |||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 510483 / Symmetry type: POINT | |||||||||||||||||||||||||||
| Atomic model building | B value: 150 / Protocol: AB INITIO MODEL / Space: RECIPROCAL | |||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6MSM Accession code: 6MSM / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller











 PDBj
PDBj














