+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 6k5t | ||||||
|---|---|---|---|---|---|---|---|
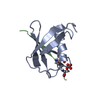
| タイトル | Complex of SUMO1 and phosphorylated hcmv protein IE2 | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | PROTEIN BINDING/TRANSCRIPTION / Complex / VIRAL PROTEIN / PROTEIN BINDING-TRANSCRIPTION complex | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報symbiont-mediated perturbation of host cell cycle G0/G1 transition checkpoint / DNA-templated viral transcription / negative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) / negative regulation of delayed rectifier potassium channel activity ...symbiont-mediated perturbation of host cell cycle G0/G1 transition checkpoint / DNA-templated viral transcription / negative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) / negative regulation of delayed rectifier potassium channel activity / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / negative regulation of DNA binding / negative regulation of action potential / PML body organization / nuclear stress granule / small protein activating enzyme binding / SUMOylation of immune response proteins / SUMOylation of DNA methylation proteins / SUMOylation of SUMOylation proteins / regulation of calcium ion transmembrane transport / XY body / Maturation of nucleoprotein / SUMOylation of RNA binding proteins / bidirectional double-stranded viral DNA replication / regulation of cardiac muscle cell contraction / Postmitotic nuclear pore complex (NPC) reformation / Maturation of nucleoprotein / negative regulation of protein import into nucleus / SUMOylation of ubiquitinylation proteins / transcription factor binding / ubiquitin-specific protease binding / cellular response to cadmium ion / symbiont-mediated perturbation of host cell cycle G1/S transition checkpoint / SUMOylation of transcription factors / ubiquitin-like protein ligase binding / roof of mouth development / SUMOylation of DNA replication proteins / protein sumoylation / potassium channel regulator activity / Regulation of IFNG signaling / postsynaptic cytosol / nuclear pore / : / transporter activator activity / SUMOylation of DNA damage response and repair proteins / presynaptic cytosol / Transcriptional and post-translational regulation of MITF-M expression and activity / SUMOylation of transcription cofactors / SUMOylation of chromatin organization proteins / SUMOylation of intracellular receptors / positive regulation of protein-containing complex assembly / PKR-mediated signaling / PML body / regulation of protein stability / protein tag activity / Formation of Incision Complex in GG-NER / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / regulation of protein localization / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / cellular response to heat / nuclear membrane / protein stabilization / nuclear speck / nuclear body / DNA repair / negative regulation of DNA-templated transcription / ubiquitin protein ligase binding / regulation of DNA-templated transcription / nucleolus / host cell nucleus / glutamatergic synapse / enzyme binding / DNA binding / RNA binding / nucleoplasm / metal ion binding / nucleus / plasma membrane / cytosol 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト)  Human cytomegalovirus (ヘルペスウイルス) Human cytomegalovirus (ヘルペスウイルス) | ||||||
| 手法 | 溶液NMR / simulated annealing | ||||||
 データ登録者 データ登録者 | Tripathi, V. / Chatterjee, K.S. | ||||||
 引用 引用 |  ジャーナル: J.Biol.Chem. / 年: 2019 ジャーナル: J.Biol.Chem. / 年: 2019タイトル: Casein kinase-2-mediated phosphorylation increases the SUMO-dependent activity of the cytomegalovirus transactivator IE2. 著者: Tripathi, V. / Chatterjee, K.S. / Das, R. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  6k5t.cif.gz 6k5t.cif.gz | 552.3 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb6k5t.ent.gz pdb6k5t.ent.gz | 459.8 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  6k5t.json.gz 6k5t.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/k5/6k5t https://data.pdbj.org/pub/pdb/validation_reports/k5/6k5t ftp://data.pdbj.org/pub/pdb/validation_reports/k5/6k5t ftp://data.pdbj.org/pub/pdb/validation_reports/k5/6k5t | HTTPS FTP |
|---|
-関連構造データ
| 関連構造データ |  6k5rC C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| その他のデータベース |
|
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR アンサンブル |
|
- 要素
要素
| #1: タンパク質 | 分子量: 8970.248 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / 発現宿主: Homo sapiens (ヒト) / 遺伝子: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / 発現宿主:  |
|---|---|
| #2: タンパク質・ペプチド | 分子量: 1369.241 Da / 分子数: 1 / 由来タイプ: 合成 由来: (合成)   Human cytomegalovirus (strain AD169) (ヘルペスウイルス) Human cytomegalovirus (strain AD169) (ヘルペスウイルス)参照: UniProt: P19893 |
| Has protein modification | Y |
-実験情報
-実験
| 実験 | 手法: 溶液NMR | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR実験 |
|
- 試料調製
試料調製
| 詳細 |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 試料 |
| ||||||||||||||||||||||||
| 試料状態 | イオン強度: 162.7 mM / Label: Codition_1 / pH: 7.4 / 圧: 1 atm / 温度: 298 K |
-NMR測定
| NMRスペクトロメーター | タイプ: Bruker AVANCE III / 製造業者: Bruker / モデル: AVANCE III / 磁場強度: 800 MHz |
|---|
- 解析
解析
| NMR software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 手法: simulated annealing / ソフトェア番号: 3 | ||||||||||||
| 代表構造 | 選択基準: lowest energy | ||||||||||||
| NMRアンサンブル | コンフォーマー選択の基準: structures with the lowest energy 計算したコンフォーマーの数: 200 / 登録したコンフォーマーの数: 20 |
 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj















 HSQC
HSQC