[English] 日本語
 Yorodumi
Yorodumi- PDB-5vjb: Guanidine-II riboswitch P2 hairpin dimer with 5-bromoU substituti... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5vjb | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
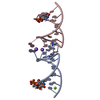
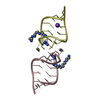
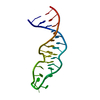
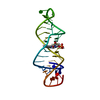
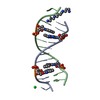
| Title | Guanidine-II riboswitch P2 hairpin dimer with 5-bromoU substitution from Pseudomonas aeruginosa | ||||||||||||||||||||||||||||
 Components Components | RNA (5'-R(* Keywords KeywordsRNA / guanidine / riboswitch / kissing loop / hairpin | Function / homology | GUANIDINE / SPERMINE / RNA / RNA (> 10) |  Function and homology information Function and homology informationBiological species |  Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.1 Å SAD / Resolution: 2.1 Å  Authors AuthorsReiss, C.W. / Strobel, S.A. | Funding support | |  United States, 1items United States, 1items
 Citation Citation Journal: RNA / Year: 2017 Journal: RNA / Year: 2017Title: Structural basis for ligand binding to the guanidine-II riboswitch. Authors: Reiss, C.W. / Strobel, S.A. History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5vjb.cif.gz 5vjb.cif.gz | 49.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5vjb.ent.gz pdb5vjb.ent.gz | 35.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5vjb.json.gz 5vjb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5vjb_validation.pdf.gz 5vjb_validation.pdf.gz | 439.5 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5vjb_full_validation.pdf.gz 5vjb_full_validation.pdf.gz | 444.3 KB | Display | |
| Data in XML |  5vjb_validation.xml.gz 5vjb_validation.xml.gz | 6.3 KB | Display | |
| Data in CIF |  5vjb_validation.cif.gz 5vjb_validation.cif.gz | 7.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vj/5vjb https://data.pdbj.org/pub/pdb/validation_reports/vj/5vjb ftp://data.pdbj.org/pub/pdb/validation_reports/vj/5vjb ftp://data.pdbj.org/pub/pdb/validation_reports/vj/5vjb | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: RNA chain | Mass: 5246.047 Da / Num. of mol.: 4 / Source method: obtained synthetically / Source: (synth.)  #2: Chemical | ChemComp-GAI / #3: Chemical | ChemComp-SPM / #4: Chemical | ChemComp-MG / | #5: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.79 % |
|---|---|
| Crystal grow | Temperature: 296.15 K / Method: microbatch / pH: 5.6 Details: 1:1 ratio of 200uM RNA in crystallization buffer (10 mM MgCl2, 10 mM KCl, 10 mM HEPES-KOH, pH 7.5, and 40 mM guanidine) and crystallization reagent (45% MPD, 50 mM MES, pH 5.6, 4 mM NaCl, 40 ...Details: 1:1 ratio of 200uM RNA in crystallization buffer (10 mM MgCl2, 10 mM KCl, 10 mM HEPES-KOH, pH 7.5, and 40 mM guanidine) and crystallization reagent (45% MPD, 50 mM MES, pH 5.6, 4 mM NaCl, 40 mM KCl, and 12 mM spermine) |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9193 Å / Beamline: 24-ID-C / Wavelength: 0.9193 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Apr 7, 2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9193 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.1→40 Å / Num. obs: 25445 / % possible obs: 99.9 % / Redundancy: 6.8 % / Rmerge(I) obs: 0.068 / Rpim(I) all: 0.028 / Rrim(I) all: 0.073 / Χ2: 1.027 / Net I/σ(I): 6.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
| Phasing | Method:  SAD SAD |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 2.1→40 Å / Cor.coef. Fo:Fc: 0.963 / Cor.coef. Fo:Fc free: 0.971 / SU B: 4.844 / SU ML: 0.127 / SU R Cruickshank DPI: 0.2048 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.205 / ESU R Free: 0.168 SAD / Resolution: 2.1→40 Å / Cor.coef. Fo:Fc: 0.963 / Cor.coef. Fo:Fc free: 0.971 / SU B: 4.844 / SU ML: 0.127 / SU R Cruickshank DPI: 0.2048 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.205 / ESU R Free: 0.168 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 102.46 Å2 / Biso mean: 40.534 Å2 / Biso min: 29.51 Å2
| ||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.1→40 Å
| ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.07→2.124 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller








 PDBj
PDBj