[English] 日本語
 Yorodumi
Yorodumi- PDB-5lcf: VIM-2 metallo-beta-lactamase in complex with 3-oxo-2-phenylisoind... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5lcf | ||||||
|---|---|---|---|---|---|---|---|
| Title | VIM-2 metallo-beta-lactamase in complex with 3-oxo-2-phenylisoindoline-4-carboxylic acid (compound 30) | ||||||
 Components Components | Metallo-beta-lactamase VIM-2 | ||||||
 Keywords Keywords | HYDROLASE / metallo-beta-lactamase / inhibitor / complex / antibiotic resistance | ||||||
| Function / homology |  Function and homology information Function and homology informationantibiotic catabolic process / beta-lactamase / periplasmic space / hydrolase activity / response to antibiotic / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.86 Å molecular replacement / Resolution: 1.86 Å | ||||||
 Authors Authors | Li, G.-B. / Brem, J. / McDonough, M.A. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Chem Sci / Year: 2017 Journal: Chem Sci / Year: 2017Title: NMR-filtered virtual screening leads to non-metal chelating metallo-beta-lactamase inhibitors. Authors: Li, G.B. / Abboud, M.I. / Brem, J. / Someya, H. / Lohans, C.T. / Yang, S.Y. / Spencer, J. / Wareham, D.W. / McDonough, M.A. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5lcf.cif.gz 5lcf.cif.gz | 105.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5lcf.ent.gz pdb5lcf.ent.gz | 78.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5lcf.json.gz 5lcf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lc/5lcf https://data.pdbj.org/pub/pdb/validation_reports/lc/5lcf ftp://data.pdbj.org/pub/pdb/validation_reports/lc/5lcf ftp://data.pdbj.org/pub/pdb/validation_reports/lc/5lcf | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5lcaC  5lchC  5le1C  5lm6C  4c1dS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 28352.836 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: blaVIM-2, bla vim-2, bla-VIM-2, blasVIM-2, blaVIM2, blm, VIM-2, vim-2, PAERUG_P32_London_17_VIM_2_10_11_06255 Plasmid: OPINF / Details (production host): PLASMID DERIVED NON-GENOMIC / Production host:  |
|---|
-Non-polymers , 5 types, 253 molecules 

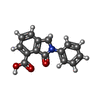






| #2: Chemical | | #3: Chemical | ChemComp-MG / | #4: Chemical | ChemComp-6TJ / | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.83 Å3/Da / Density % sol: 32.7 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 0.1 M Magnesium Formate, 21% (v/v) Polyethylene glycol 3350, 5 mM 3-oxo-2-phenylisoindoline-4-carboxylic acid, 16 mg/mL protein |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.542 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.542 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU SATURN 944+ / Detector: CCD / Date: Jun 13, 2016 / Details: OSMIC HF | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.542 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.86→50 Å / Num. obs: 16377 / % possible obs: 99.6 % / Redundancy: 6.7 % / Biso Wilson estimate: 14.94 Å2 / Rmerge(I) obs: 0.058 / Rpim(I) all: 0.024 / Rrim(I) all: 0.062 / Χ2: 1.06 / Net I/av σ(I): 26.418 / Net I/σ(I): 18.2 / Num. measured all: 110191 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4C1D Resolution: 1.86→24.055 Å / SU ML: 0.15 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 14.06
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 52.86 Å2 / Biso mean: 15.2723 Å2 / Biso min: 5.02 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.86→24.055 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 12
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





