[English] 日本語
 Yorodumi
Yorodumi- PDB-5elj: Isoform-specific inhibition of SUMO-dependent protein-protein int... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5elj | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
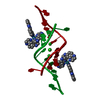
| Title | Isoform-specific inhibition of SUMO-dependent protein-protein interactions | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | SIGNALING PROTEIN / Ubiquitin / Sumoylation | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) / negative regulation of delayed rectifier potassium channel activity / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / negative regulation of DNA binding ...negative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) / negative regulation of delayed rectifier potassium channel activity / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / negative regulation of DNA binding / negative regulation of action potential / PML body organization / nuclear stress granule / small protein activating enzyme binding / SUMOylation of immune response proteins / SUMOylation of SUMOylation proteins / SUMOylation of DNA methylation proteins / regulation of calcium ion transmembrane transport / Maturation of nucleoprotein / SUMOylation of RNA binding proteins / XY body / regulation of cardiac muscle cell contraction / Postmitotic nuclear pore complex (NPC) reformation / Maturation of nucleoprotein / negative regulation of protein import into nucleus / SUMOylation of ubiquitinylation proteins / transcription factor binding / ubiquitin-specific protease binding / cellular response to cadmium ion / SUMOylation of transcription factors / ubiquitin-like protein ligase binding / roof of mouth development / SUMOylation of DNA replication proteins / protein sumoylation / potassium channel regulator activity / Regulation of IFNG signaling / postsynaptic cytosol / nuclear pore / : / transporter activator activity / SUMOylation of DNA damage response and repair proteins / presynaptic cytosol / Transcriptional and post-translational regulation of MITF-M expression and activity / SUMOylation of transcription cofactors / SUMOylation of chromatin organization proteins / SUMOylation of intracellular receptors / positive regulation of protein-containing complex assembly / regulation of protein stability / PKR-mediated signaling / PML body / protein tag activity / Formation of Incision Complex in GG-NER / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / regulation of protein localization / cellular response to heat / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / nuclear membrane / protein stabilization / nuclear speck / nuclear body / DNA repair / negative regulation of DNA-templated transcription / ubiquitin protein ligase binding / nucleolus / glutamatergic synapse / enzyme binding / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species | synthetic construct (others) Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.983 Å MOLECULAR REPLACEMENT / Resolution: 1.983 Å | ||||||||||||
 Authors Authors | Hughes, D.J. / Tiede, C. / Hall, N. / Tang, A.A.S. / Trinh, C.H. / Zajac, K. / Mandal, U. / Howell, G. / Edwards, T.A. / McPherson, M.J. ...Hughes, D.J. / Tiede, C. / Hall, N. / Tang, A.A.S. / Trinh, C.H. / Zajac, K. / Mandal, U. / Howell, G. / Edwards, T.A. / McPherson, M.J. / Tomlinson, D.C. / Whitehouse, A. | ||||||||||||
| Funding support |  United Kingdom, 3items United Kingdom, 3items
| ||||||||||||
 Citation Citation |  Journal: Sci Signal / Year: 2017 Journal: Sci Signal / Year: 2017Title: Generation of specific inhibitors of SUMO-1- and SUMO-2/3-mediated protein-protein interactions using Affimer (Adhiron) technology. Authors: Hughes, D.J. / Tiede, C. / Penswick, N. / Tang, A.A. / Trinh, C.H. / Mandal, U. / Zajac, K.Z. / Gaule, T. / Howell, G. / Edwards, T.A. / Duan, J. / Feyfant, E. / McPherson, M.J. / Tomlinson, ...Authors: Hughes, D.J. / Tiede, C. / Penswick, N. / Tang, A.A. / Trinh, C.H. / Mandal, U. / Zajac, K.Z. / Gaule, T. / Howell, G. / Edwards, T.A. / Duan, J. / Feyfant, E. / McPherson, M.J. / Tomlinson, D.C. / Whitehouse, A. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5elj.cif.gz 5elj.cif.gz | 85.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5elj.ent.gz pdb5elj.ent.gz | 63.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5elj.json.gz 5elj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/el/5elj https://data.pdbj.org/pub/pdb/validation_reports/el/5elj ftp://data.pdbj.org/pub/pdb/validation_reports/el/5elj ftp://data.pdbj.org/pub/pdb/validation_reports/el/5elj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5eluC  5eqlC  2uyzS  4n6tS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 13252.120 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Plasmid: PET11 / Production host:  |
|---|---|
| #2: Protein | Mass: 9686.066 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / Plasmid: PET11 / Production host: Homo sapiens (human) / Gene: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / Plasmid: PET11 / Production host:  |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.46 Å3/Da / Density % sol: 49.98 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 0.1 M Hepes sodium salt, 10% w/v polyethylene glycol 20000 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.987 Å / Beamline: I04 / Wavelength: 0.987 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Dec 6, 2013 |
| Radiation | Monochromator: SAGITALLY FOCUSED Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.987 Å / Relative weight: 1 |
| Reflection | Resolution: 1.98→70.7 Å / Num. obs: 15526 / % possible obs: 99.4 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 2 / Redundancy: 12.3 % / Biso Wilson estimate: 44.1 Å2 / Rmerge(I) obs: 0.061 / Net I/σ(I): 19.3 |
| Reflection shell | Resolution: 1.98→2.03 Å / Redundancy: 13 % / Rmerge(I) obs: 0.438 / Mean I/σ(I) obs: 5.2 / % possible all: 99.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRIES 2UYZ and 4N6T Resolution: 1.983→54.001 Å / SU ML: 0.19 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 26.49 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.983→54.001 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj

















