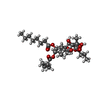
Entry Database : PDB / ID : 5a3sTitle Crystal structure of the (SR) Calcium ATPase E2-vanadate complex bound to thapsigargin and TNP-ATP SARCOPLASMIC RETICULUM CALCIUM ATPASE 1 MOLECULE SARCOPLASMIC/ENDOPLASMIC RETICULUM CALCIUM ATPASE 1 Keywords / / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species ORYCTOLAGUS CUNICULUS (rabbit)Method / / / Resolution : 3.3 Å Authors Clausen, J.D. / Bublitz, M. / Arnou, B. / Olesen, C. / Andersen, J.P. / Moller, J.V. / Nissen, P. Journal : Structure / Year : 2016Title : Crystal Structure of the Vanadate-Inhibited Ca(2+)-ATPase.Authors : Clausen, J.D. / Bublitz, M. / Arnou, B. / Olesen, C. / Andersen, J.P. / Moller, J.V. / Nissen, P. History Deposition Jun 3, 2015 Deposition site / Processing site Revision 1.0 Apr 13, 2016 Provider / Type Revision 1.1 Apr 20, 2016 Group Revision 1.2 May 15, 2019 Group Data collection / Derived calculations ... Data collection / Derived calculations / Experimental preparation / Other Category exptl_crystal_grow / pdbx_database_proc ... exptl_crystal_grow / pdbx_database_proc / pdbx_database_status / struct_biol / struct_conn Item _exptl_crystal_grow.method / _exptl_crystal_grow.temp ... _exptl_crystal_grow.method / _exptl_crystal_grow.temp / _pdbx_database_status.recvd_author_approval / _struct_conn.pdbx_leaving_atom_flag Revision 1.3 Oct 23, 2019 Group / Database references / Other / Category / struct_ref_seq_difItem / _struct_ref_seq_dif.detailsRevision 1.4 Jan 10, 2024 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description / Structure summary Category chem_comp / chem_comp_atom ... chem_comp / chem_comp_atom / chem_comp_bond / database_2 / entity / pdbx_entity_nonpoly / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn / struct_site Item _chem_comp.name / _database_2.pdbx_DOI ... _chem_comp.name / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _entity.pdbx_description / _pdbx_entity_nonpoly.name / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_comp_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr2_label_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 1.5 Oct 23, 2024 Group / Category / pdbx_modification_feature / Item
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.3 Å
MOLECULAR REPLACEMENT / Resolution: 3.3 Å  Authors
Authors Citation
Citation Journal: Structure / Year: 2016
Journal: Structure / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5a3s.cif.gz
5a3s.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5a3s.ent.gz
pdb5a3s.ent.gz PDB format
PDB format 5a3s.json.gz
5a3s.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 5a3s_validation.pdf.gz
5a3s_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 5a3s_full_validation.pdf.gz
5a3s_full_validation.pdf.gz 5a3s_validation.xml.gz
5a3s_validation.xml.gz 5a3s_validation.cif.gz
5a3s_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/a3/5a3s
https://data.pdbj.org/pub/pdb/validation_reports/a3/5a3s ftp://data.pdbj.org/pub/pdb/validation_reports/a3/5a3s
ftp://data.pdbj.org/pub/pdb/validation_reports/a3/5a3s

 Links
Links Assembly
Assembly


 Components
Components











 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SLS
SLS  / Beamline: X06SA / Wavelength: 0.90501
/ Beamline: X06SA / Wavelength: 0.90501  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller








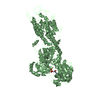













 PDBj
PDBj







