| Entry | Database: PDB / ID: 6yso
|
|---|
| Title | Crystal structure of the (SR) Ca2+-ATPase solved by vanadium SAD phasing |
|---|
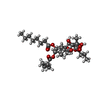
 Components Components | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 |
|---|
 Keywords Keywords | HYDROLASE / P-type ATPase / Vanadate / SAD phasing |
|---|
| Function / homology |  Function and homology information Function and homology information
positive regulation of cardiac muscle cell contraction / positive regulation of calcium ion import into sarcoplasmic reticulum / positive regulation of ATPase-coupled calcium transmembrane transporter activity / positive regulation of fast-twitch skeletal muscle fiber contraction / H zone / regulation of striated muscle contraction / calcium ion import into sarcoplasmic reticulum / negative regulation of striated muscle contraction / P-type Ca2+ transporter / P-type calcium transporter activity ...positive regulation of cardiac muscle cell contraction / positive regulation of calcium ion import into sarcoplasmic reticulum / positive regulation of ATPase-coupled calcium transmembrane transporter activity / positive regulation of fast-twitch skeletal muscle fiber contraction / H zone / regulation of striated muscle contraction / calcium ion import into sarcoplasmic reticulum / negative regulation of striated muscle contraction / P-type Ca2+ transporter / P-type calcium transporter activity / I band / endoplasmic reticulum-Golgi intermediate compartment / sarcoplasmic reticulum membrane / sarcoplasmic reticulum / calcium ion transport / calcium ion binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm / endoplasmic reticulum / ATP hydrolysis activity / ATP binding / membraneSimilarity search - Function P-type ATPase, subfamily IIA, SERCA-type / Calcium-transporting ATPase, cytoplasmic domain N / Calcium-transporting ATPase, cytoplasmic domain N / haloacid dehalogenase-like hydrolase / Cation-transporting P-type ATPase, C-terminal / Cation transporting ATPase, C-terminus / Cation transporter/ATPase, N-terminus / Cation-transporting P-type ATPase, N-terminal / Cation transporter/ATPase, N-terminus / P-type ATPase, cytoplasmic domain N ...P-type ATPase, subfamily IIA, SERCA-type / Calcium-transporting ATPase, cytoplasmic domain N / Calcium-transporting ATPase, cytoplasmic domain N / haloacid dehalogenase-like hydrolase / Cation-transporting P-type ATPase, C-terminal / Cation transporting ATPase, C-terminus / Cation transporter/ATPase, N-terminus / Cation-transporting P-type ATPase, N-terminal / Cation transporter/ATPase, N-terminus / P-type ATPase, cytoplasmic domain N / P-type ATPase actuator domain / P-type ATPase, haloacid dehalogenase domain / P-type ATPase, phosphorylation site / P-type ATPase, cytoplasmic domain N / E1-E2 ATPases phosphorylation site. / P-type ATPase, A domain superfamily / P-type ATPase / P-type ATPase, transmembrane domain superfamily / haloacid dehalogenase-like hydrolase / HAD superfamily / HAD-like superfamily / 3-Layer(aba) Sandwich / Alpha BetaSimilarity search - Domain/homology |
|---|
| Biological species |   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 3.13 Å SAD / Resolution: 3.13 Å |
|---|
 Authors Authors | El Omari, K. / Mohamad, N. / Bountra, K. / Duman, R. / Romano, M. / Schlegel, K. / Kwong, H. / Mykhaylyk, V. / Olesen, C.E. / Moller, J.V. ...El Omari, K. / Mohamad, N. / Bountra, K. / Duman, R. / Romano, M. / Schlegel, K. / Kwong, H. / Mykhaylyk, V. / Olesen, C.E. / Moller, J.V. / Bublitz, M. / Beis, K. / Wagner, A. |
|---|
 Citation Citation |  Journal: Iucrj / Year: 2020 Journal: Iucrj / Year: 2020
Title: Experimental phasing with vanadium and application to nucleotide-binding membrane proteins.
Authors: El Omari, K. / Mohamad, N. / Bountra, K. / Duman, R. / Romano, M. / Schlegel, K. / Kwong, H.S. / Mykhaylyk, V. / Olesen, C. / Moller, J.V. / Bublitz, M. / Beis, K. / Wagner, A. |
|---|
| History | | Deposition | Apr 22, 2020 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Nov 4, 2020 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Dec 2, 2020 | Group: Database references / Category: citation / citation_author
Item: _citation.journal_volume / _citation.page_first ..._citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_PubMed / _citation.title / _citation_author.name |
|---|
| Revision 1.2 | May 15, 2024 | Group: Data collection / Database references / Category: chem_comp_atom / chem_comp_bond / database_2
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SAD / Resolution: 3.13 Å
SAD / Resolution: 3.13 Å  Authors
Authors Citation
Citation Journal: Iucrj / Year: 2020
Journal: Iucrj / Year: 2020 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6yso.cif.gz
6yso.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6yso.ent.gz
pdb6yso.ent.gz PDB format
PDB format 6yso.json.gz
6yso.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ys/6yso
https://data.pdbj.org/pub/pdb/validation_reports/ys/6yso ftp://data.pdbj.org/pub/pdb/validation_reports/ys/6yso
ftp://data.pdbj.org/pub/pdb/validation_reports/ys/6yso Links
Links Assembly
Assembly


 Components
Components











 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I23 / Wavelength: 2.26009 Å
/ Beamline: I23 / Wavelength: 2.26009 Å Processing
Processing SAD / Resolution: 3.13→64.585 Å / SU ML: 0.51 / Cross valid method: THROUGHOUT / σ(F): 1.33 / Phase error: 29.6
SAD / Resolution: 3.13→64.585 Å / SU ML: 0.51 / Cross valid method: THROUGHOUT / σ(F): 1.33 / Phase error: 29.6  Movie
Movie Controller
Controller























 PDBj
PDBj







