[English] 日本語
 Yorodumi
Yorodumi- PDB-4zy0: X-ray crystal structure of PfA-M17 in complex with hydroxamic aci... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4zy0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray crystal structure of PfA-M17 in complex with hydroxamic acid-based inhibitor 10q | ||||||
 Components Components | Probable M17 family aminopeptidase | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / M17 LEUCYL-AMINOPEPTIDASE / PROTEASE / INHIBITOR / HYDROXAMIC ACID / HYDROLASE-HYDROLASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationleucyl aminopeptidase / metalloaminopeptidase activity / manganese ion binding / proteolysis / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Drinkwater, N. / McGowan, S. | ||||||
| Funding support |  Australia, 1items Australia, 1items
| ||||||
 Citation Citation |  Journal: Eur.J.Med.Chem. / Year: 2016 Journal: Eur.J.Med.Chem. / Year: 2016Title: Potent dual inhibitors of Plasmodium falciparum M1 and M17 aminopeptidases through optimization of S1 pocket interactions. Authors: Drinkwater, N. / Vinh, N.B. / Mistry, S.N. / Bamert, R.S. / Ruggeri, C. / Holleran, J.P. / Loganathan, S. / Paiardini, A. / Charman, S.A. / Powell, A.K. / Avery, V.M. / McGowan, S. / Scammells, P.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4zy0.cif.gz 4zy0.cif.gz | 2.4 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4zy0.ent.gz pdb4zy0.ent.gz | 2 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4zy0.json.gz 4zy0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4zy0_validation.pdf.gz 4zy0_validation.pdf.gz | 3.8 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4zy0_full_validation.pdf.gz 4zy0_full_validation.pdf.gz | 3.8 MB | Display | |
| Data in XML |  4zy0_validation.xml.gz 4zy0_validation.xml.gz | 240 KB | Display | |
| Data in CIF |  4zy0_validation.cif.gz 4zy0_validation.cif.gz | 336.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zy/4zy0 https://data.pdbj.org/pub/pdb/validation_reports/zy/4zy0 ftp://data.pdbj.org/pub/pdb/validation_reports/zy/4zy0 ftp://data.pdbj.org/pub/pdb/validation_reports/zy/4zy0 | HTTPS FTP |
-Related structure data
| Related structure data |  4zw3C  4zw5C  4zw6C  4zw7C  4zw8C  4zx3C  4zx4C  4zx5C  4zx6C  4zx8C  4zx9C  4zy1C  4zy2C  4zyqC  3kqzS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 12 molecules ABCDEFGHIJKL
| #1: Protein | Mass: 57982.230 Da / Num. of mol.: 12 / Fragment: UNP residues 84-605 / Mutation: D152N,D515N,D516N Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Strain: isolate FcB1 / Columbia / Plasmid: PTRCHIS-2B / Production host:  |
|---|
-Non-polymers , 7 types, 3388 molecules 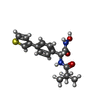












| #2: Chemical | ChemComp-4TM / #3: Chemical | ChemComp-ZN / #4: Chemical | ChemComp-CO3 / #5: Chemical | ChemComp-1PE / #6: Chemical | ChemComp-SO4 / #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.55 Å3/Da / Density % sol: 51.81 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 8.5 / Details: 40% (v/v) PEG 400, 0.1 M Tris pH 8.5, 0.2 M Li2SO4 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX2 / Wavelength: 0.9537 Å / Beamline: MX2 / Wavelength: 0.9537 Å | |||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Sep 26, 2014 | |||||||||||||||||||||||||||
| Radiation | Monochromator: DOUBLE CRYSTAL SILICON 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9537 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection | Resolution: 2.2→48.63 Å / Num. obs: 355767 / % possible obs: 100 % / Redundancy: 6.1 % / Biso Wilson estimate: 24.24 Å2 / CC1/2: 0.987 / Rmerge(I) obs: 0.406 / Rpim(I) all: 0.163 / Net I/σ(I): 5.6 / Num. measured all: 2155326 / Scaling rejects: 887 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3KQZ Resolution: 2.2→48.63 Å / FOM work R set: 0.7603 / SU ML: 0.28 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 29.99 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 114.18 Å2 / Biso mean: 29.99 Å2 / Biso min: 10.8 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.2→48.63 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 30
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 175.9693 Å / Origin y: 231.8708 Å / Origin z: -35.5712 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj









