[English] 日本語
 Yorodumi
Yorodumi- PDB-4xnx: X-ray structure of Drosophila dopamine transporter in complex wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4xnx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | X-ray structure of Drosophila dopamine transporter in complex with reboxetine | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | transport protein/inhibitor / integral membrane protein / all-alpha helical antidepressant complex / transport protein-inhibitor complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationcatecholamine uptake / Dopamine clearance from the synaptic cleft / SLC-mediated transport of neurotransmitters / circadian sleep/wake cycle / sodium:chloride symporter activity / response to odorant / cocaine binding / norepinephrine transport / dopamine:sodium symporter activity / regulation of presynaptic cytosolic calcium ion concentration ...catecholamine uptake / Dopamine clearance from the synaptic cleft / SLC-mediated transport of neurotransmitters / circadian sleep/wake cycle / sodium:chloride symporter activity / response to odorant / cocaine binding / norepinephrine transport / dopamine:sodium symporter activity / regulation of presynaptic cytosolic calcium ion concentration / dopamine transport / monoamine transmembrane transporter activity / sleep / neuronal cell body membrane / neurotransmitter transport / dopamine uptake involved in synaptic transmission / amino acid transport / sodium ion transmembrane transport / adult locomotory behavior / presynaptic membrane / axon / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3 Å MOLECULAR REPLACEMENT / Resolution: 3 Å | |||||||||
 Authors Authors | Aravind, P. / Wang, K. / Gouaux, E. | |||||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2015 Journal: Nat.Struct.Mol.Biol. / Year: 2015Title: X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Authors: Penmatsa, A. / Wang, K.H. / Gouaux, E. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4xnx.cif.gz 4xnx.cif.gz | 207.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4xnx.ent.gz pdb4xnx.ent.gz | 158.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4xnx.json.gz 4xnx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4xnx_validation.pdf.gz 4xnx_validation.pdf.gz | 1.5 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4xnx_full_validation.pdf.gz 4xnx_full_validation.pdf.gz | 1.5 MB | Display | |
| Data in XML |  4xnx_validation.xml.gz 4xnx_validation.xml.gz | 36.9 KB | Display | |
| Data in CIF |  4xnx_validation.cif.gz 4xnx_validation.cif.gz | 49.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xn/4xnx https://data.pdbj.org/pub/pdb/validation_reports/xn/4xnx ftp://data.pdbj.org/pub/pdb/validation_reports/xn/4xnx ftp://data.pdbj.org/pub/pdb/validation_reports/xn/4xnx | HTTPS FTP |
-Related structure data
| Related structure data |  4xnuC  4m48S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Antibody , 2 types, 2 molecules LH
| #2: Antibody | Mass: 23306.586 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #3: Antibody | Mass: 25921.338 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Protein / Sugars , 2 types, 2 molecules A
| #1: Protein | Mass: 60148.539 Da / Num. of mol.: 1 / Mutation: V74, L415A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: A0A0B4KEX2, UniProt: Q7K4Y6*PLUS Homo sapiens (human) / References: UniProt: A0A0B4KEX2, UniProt: Q7K4Y6*PLUS |
|---|---|
| #4: Polysaccharide | alpha-D-glucopyranose-(1-4)-beta-D-glucopyranose / beta-maltose |
-Non-polymers , 5 types, 12 molecules 


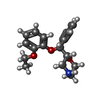





| #5: Chemical | | #6: Chemical | #7: Chemical | ChemComp-CL / | #8: Chemical | ChemComp-41X / ( | #9: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.92 Å3/Da / Density % sol: 75.02 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / Details: PEG 350 MME 38%, Glycine 0.1M / PH range: 9 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.979 Å / Beamline: 24-ID-C / Wavelength: 0.979 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Mar 15, 2013 |
| Radiation | Monochromator: Si111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 2.997→48.417 Å / Num. obs: 46863 / % possible obs: 99.4 % / Redundancy: 14.4 % / Biso Wilson estimate: 116.6 Å2 / Rmerge(I) obs: 0.11 / Net I/σ(I): 5.6 |
| Reflection shell | Resolution: 3→3.11 Å / Redundancy: 14 % / Rmerge(I) obs: 0.866 / % possible all: 99.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4M48 Resolution: 3→48.42 Å / SU ML: 0.41 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 28.6 / Stereochemistry target values: ML Details: Residue H CYS 134 and Residue H THR 138 are not properly linked: distance between C and N is 9.24.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 110.3 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3→48.42 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller































 PDBj
PDBj