[English] 日本語
 Yorodumi
Yorodumi- PDB-4rrr: K121M mutant of N-terminal editing domain of threonyl-tRNA synthe... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4rrr | ||||||
|---|---|---|---|---|---|---|---|
| Title | K121M mutant of N-terminal editing domain of threonyl-tRNA synthetase from Pyrococcus abyssi with L-Thr3AA | ||||||
 Components Components | Threonine--tRNA ligase | ||||||
 Keywords Keywords | LIGASE / DTD-like fold / Proofreading | ||||||
| Function / homology |  Function and homology information Function and homology informationthreonine-tRNA ligase / threonyl-tRNA aminoacylation / threonine-tRNA ligase activity / tRNA binding / zinc ion binding / ATP binding / cytoplasm Similarity search - Function | ||||||
| Biological species |   Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.86 Å MOLECULAR REPLACEMENT / Resolution: 1.86 Å | ||||||
 Authors Authors | Hussain, T. / Kamarthapu, V. / Sankaranarayanan, R. | ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2015 Journal: Nat Commun / Year: 2015Title: Specificity and catalysis hardwired at the RNA-protein interface in a translational proofreading enzyme. Authors: Ahmad, S. / Muthukumar, S. / Kuncha, S.K. / Routh, S.B. / Yerabham, A.S. / Hussain, T. / Kamarthapu, V. / Kruparani, S.P. / Sankaranarayanan, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4rrr.cif.gz 4rrr.cif.gz | 74 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4rrr.ent.gz pdb4rrr.ent.gz | 54.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4rrr.json.gz 4rrr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rr/4rrr https://data.pdbj.org/pub/pdb/validation_reports/rr/4rrr ftp://data.pdbj.org/pub/pdb/validation_reports/rr/4rrr ftp://data.pdbj.org/pub/pdb/validation_reports/rr/4rrr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4rr6C  4rr7C  4rr8C 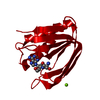 4rr9C  4rraC  4rrbC  4rrcC  4rrdC  4rrfC  4rrgC  4rrhC  4rriC  4rrjC  4rrkC  4rrlC  4rrmC  4rrqC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 16740.328 Da / Num. of mol.: 2 / Fragment: UNP residues 1-147 / Mutation: K121M Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus abyssi GE5 (archaea) / Strain: GE5 / Orsay / Gene: thrS, PYRAB13430, PAB1490 / Production host: Pyrococcus abyssi GE5 (archaea) / Strain: GE5 / Orsay / Gene: thrS, PYRAB13430, PAB1490 / Production host:  #2: Chemical | #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.84 Å3/Da / Density % sol: 33.16 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: BisTris, NaCl, PEG3350, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH3R / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU RUH3R / Wavelength: 1.5418 Å |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: Aug 22, 2009 |
| Radiation | Monochromator: Mirrors / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.86→25 Å / Num. all: 20823 / Num. obs: 20636 / % possible obs: 99.1 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.86→25 Å MOLECULAR REPLACEMENT / Resolution: 1.86→25 Å
| |||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.86→25 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj